��Ŀ����
����Ŀ������β����Ⱦ�ѳ�Ϊһ����������ȼ���У������������������һ��������Ͻ������ף������õ����ȼ������ȼ��ʹ�������¶�Ѹ�����ߣ���������������ͣ�����ѹ���ƶ�������ȼ��ʱ�������к�����ͨ��β���ų����Ӷ���Ⱦ������Ϊ�����ۣ��������͵ijɷ�ȫ��Ϊ���飬������������������������Ϊ20%������Ϊ��������ش��������⣺
(1)����������������������Ϊa��Ҫʹ������ȫȼ�գ���a�����ֵΪ_____________��
(2)�����������������ȴ���a�����ֵʱ����Ⱦ�������к���������������______________________��
(3)������������������С��a�����ֵʱ����β����������Ⱦ�������к������������_____________������������Ļ�ѧ����ʽ��____________________________��
(4)������������������С��a�����ֵʱ���������Ƕ�������������Ҳ�бף���������������������������������������ȫȼ�գ������Դ�����ʡ�����ٳ�һ�����������أ�_________________________________________________________________��
���𰸡� 0.016 CO NO��NO2N2+O2![]() 2NO 2NO+O2=2NO2 �������������������ʱ���ߵ������϶�����������������
2NO 2NO+O2=2NO2 �������������������ʱ���ߵ������϶�����������������
�����������������(1)�������ȼ�շ���ʽ2C8H18+25O2![]() 16CO2+18H2O��֪�������������������ʵ���֮��Ϊ2��25ʱǡ�÷�Ӧ�������ڿ����еĺ���20%��
16CO2+18H2O��֪�������������������ʵ���֮��Ϊ2��25ʱǡ�÷�Ӧ�������ڿ����еĺ���20%��
(2)������������������ȴ���0.016ʱ�����������㣬���鲻����ȫȼ�գ��������������CO���塣
(3)�������������������С��0.016ʱ��������������������ȫȼ������CO2��ˮ�� CO2�����ڴ�����Ⱦ��ӿ����ĽǶ���˼������Ⱦ�������к�����������ǵ��������
(4)�����������������ʣ�����ʱ���ߵ������϶���
������(1)�������ȼ�շ���ʽ2C8H18+25O2![]() 16CO2+18H2O��֪�������������������ʵ���֮��Ϊ2��25ʱǡ�÷�Ӧ���������������������������Ǵ���ͬ��ͬѹ�������£��ʴˣ��������������������Ϊ2��25ʱǡ�÷�Ӧ��a�����ֵӦΪ
16CO2+18H2O��֪�������������������ʵ���֮��Ϊ2��25ʱǡ�÷�Ӧ���������������������������Ǵ���ͬ��ͬѹ�������£��ʴˣ��������������������Ϊ2��25ʱǡ�÷�Ӧ��a�����ֵӦΪ![]() 0.016��
0.016��
(2)������������������ȴ���0.016ʱ�����������㣬���鲻����ȫȼ�գ������������CO���塣
(3)�������������������С��0.016ʱ��������������������ȫȼ������CO2��ˮ�� CO2�����ڴ�����Ⱦ��ӿ����ĽǶ���˼������Ⱦ�������к�����������ǵ�����������Ӧ���ɵ�NO��NO2������ʽΪN2+O2![]() 2NO��
2NO��
(4)���������࣬����ʣ�����ʱ���ߵ������϶�������������������

����Ŀ��(NH4)2Fe(SO4)26H2O(Ī���Σ�dz��ɫ��ʽ��392)�ڶ��������г������궨������ء��ظ���ص���Һ�ı����ʣ���������ѧ�Լ���ҽҩ�Լ�����ұ�𡢵�Ƶȡ�
�ش��������⣺
��1��Ī�����ڿ����б����������ȶ���������¶���ڿ�����Ҳ����ʣ�����Ī�����Ƿ���ʵ��Լ���________��
��2��ȷ��ȡmg������Ī���Σ�����ƿ�м���20mLˮ����ܽ⣬��ij����K2Cr2O7��Һ�ζ����յ㣮�ظ�����3�Σ�����й��������£�
ʵ����� | ��ʼ����/mL | �յ����/mL |
I | 2.50 | 22.58 |
�� | 1.00 | 23.12 |
�� | 0.00 | 19.92 |
��K2Cr2O7��ҺӦ�÷���________ʽ�ζ����У�
��д���ζ������з�Ӧ�����ӷ���ʽ��________��
������K2Cr2O7��Һ�����ʵ���Ũ��Ϊ________mol/L(�ú�M�Ĵ���ʽ��ʾ)
��3��ij������ͨ��ʵ�����Ī���ξ������ʱ�ķֽ���
����ͬѧ������룺�ֽ���������N2��Fe2O3��SO3��H2O�������ʣ����Ƿ�ͬ�Ⲣ˵�����ɣ�________��
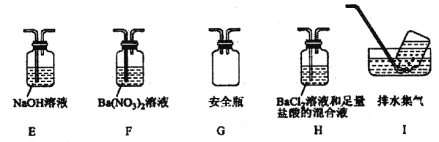
����ͬѧ�������ͼװ�ã�����Aװ���еĹ����Ϊ����ɫ�����������к���________��Cװ���к�ɫ��ȥ��˵����������к���________��Cװ�ú�Ӧ����β������װ��D��D��ʢ�е��Լ�������________(дһ�ּ���)��

����ͬѧ����������װ��֤���ֽ�����к��а�����ֻ�����B��C�е��Լ����ɣ����������Լ�ΪB________��C________��
����ͬѧ��ΪĪ���ηֽ���ܻ�����N2��SO3���������װ����ͼ2��ѡ���Ҫ��װ�ü���֤��������ȷ������˳�������������A��________��
����Ŀ������1����25�桢101kPa�£�1 g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.68 kJ����״���ȼ���ȵ��Ȼ�ѧ����ʽΪ��_________________________________
���������к͵ζ��ⶨij������Һ��Ũ�ȣ��й����ݼ�¼���£�
�ζ���� | ����Һ���(mL) | �������ռ���Һ�����(mL) | ||
�ζ�ǰ | �ζ��� | ���ĵ���� | ||
1 | 25.00 | 0.50 | 25.12 | 24.62 |
2 | 25.00 | / | / | / |
3 | 25.00 | 5.00 | 29.58 | 24.58 |
��2��ʵ������ʯ��ͷ�̪����ָʾ������ʵ��Ӧѡ��_______��ָʾ������______ʽ�ζ���ʢװ0.2500 mol/L�ռ���Һ����ƿ��װ��25.00mL����������Һ��
��3����ͼ��ʾ�ڶ��εζ�ʱ50 mL�ζ�����ǰ��Һ���λ�á��ôεζ����ñ��ռ���Һ���Ϊ_______mL��

��4�������������ݣ�����������������ʵ���Ũ�ȣ�ע�Ᵽ�����ʵ���Ч���֣���c (HCl) = _____________��
��5������ʱ�����ζ�ǰ���ӣ��ζ����ӣ����ʹ���ղⶨ���_________��(������Ӱ��������ƫ��������ƫ����)
����Ŀ������ͼ��ʾװ�ü�����ϩʱ����Ҫ���ӵ���
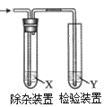
��ϩ���Ʊ� | �Լ�X | �Լ�Y | |
A | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | KMnO4������Һ |
B | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | Br2��CCl4��Һ |
C | CH3CH2OH��ŨH2SO4������170�� | NaOH��Һ | KMnO4������Һ |
D | CH3CH2OH��ŨH2SO4������170�� | NaOH��Һ | Br2��ˮ��Һ |
A. A B. B C. C D. D

