��Ŀ����
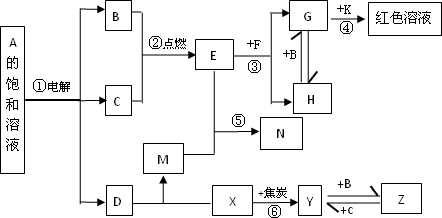
����A��M����ͼ��ʾ��ת����ϵ������������ʡ�ԣ�������C��D���� X��Y��Z������Ԫ����ɵĻ����X��Y��Z��ԭ�������������������ڱ���X ��ԭ�Ӱ뾶��С��Y��Z��ԭ������������֮��Ϊ
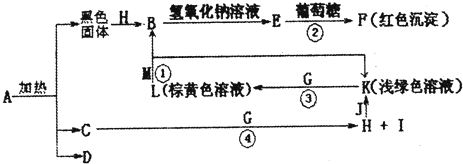
10��DΪ��ɫ�ǿ�ȼ�����壬 GΪ����ɫ���嵥�ʣ�J��MΪ������I��Ư�����ã���Ӧ�ٳ���������ӡˢ�� ·�塣
10��DΪ��ɫ�ǿ�ȼ�����壬 GΪ����ɫ���嵥�ʣ�J��MΪ������I��Ư�����ã���Ӧ�ٳ���������ӡˢ�� ·�塣
��ش��������⣺
(1)�Ƚ�Y��Z��ԭ�Ӱ뾶��С��____>____����Ԫ�ط��ţ���
(2)д��A�Ļ�ѧʽ��________��D�ĵ���ʽ��________��
(3)�ٳ���Ӧ���������е�һ��Ӧ��ʵ����____________��
(4)д����Ӧ�ܵĻ�ѧ����ʽ��____________________��
(5)��֪F����ϡ���ᣬ��Һ�����ɫ�����ų���ɫ���壬��д���÷�Ӧ�Ļ�ѧ����ʽ��_____________��
(6)�о�����������D��һ�������¿ɱ���ԭΪ��Ө���ľ���N����ṹ��ԭ�ӵ�����Ϊ�������壬��д��N��������ͬ������������ƣ�________�� ________��________
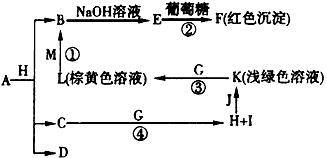
(1)�Ƚ�Y��Z��ԭ�Ӱ뾶��С��____>____����Ԫ�ط��ţ���
(2)д��A�Ļ�ѧʽ��________��D�ĵ���ʽ��________��
(3)�ٳ���Ӧ���������е�һ��Ӧ��ʵ����____________��
(4)д����Ӧ�ܵĻ�ѧ����ʽ��____________________��
(5)��֪F����ϡ���ᣬ��Һ�����ɫ�����ų���ɫ���壬��д���÷�Ӧ�Ļ�ѧ����ʽ��_____________��
(6)�о�����������D��һ�������¿ɱ���ԭΪ��Ө���ľ���N����ṹ��ԭ�ӵ�����Ϊ�������壬��д��N��������ͬ������������ƣ�________�� ________��________
(1)C��O
(2)Cu2(OH)2CO3[Cu(OH)2��CuCO3]��CuCO3��
(3)ҽѧ�Ͽ��������Ӧ������Һ�е�������
(4)Cl2+H2O=HCl+HClO
(5)3Cu2O+14HNO3=6Cu(NO3)2+2NO��+7H2O
(6)���ʯ��ʯī������ϩ��C60��̼���ܵȣ�
(2)Cu2(OH)2CO3[Cu(OH)2��CuCO3]��CuCO3��

(3)ҽѧ�Ͽ��������Ӧ������Һ�е�������
(4)Cl2+H2O=HCl+HClO
(5)3Cu2O+14HNO3=6Cu(NO3)2+2NO��+7H2O
(6)���ʯ��ʯī������ϩ��C60��̼���ܵȣ�

��ϰ��ϵ�д�
�����Ŀ

 Al��OH��3+3H+
Al��OH��3+3H+