��Ŀ����
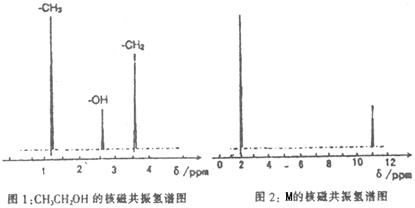
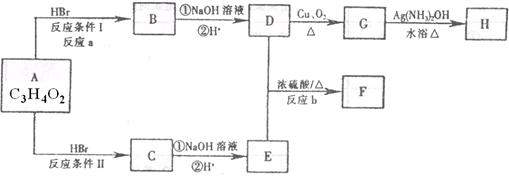
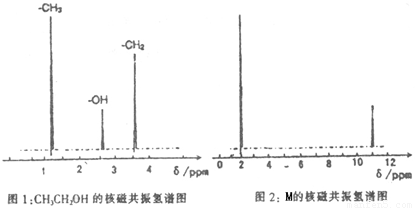
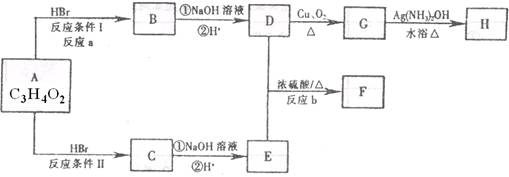
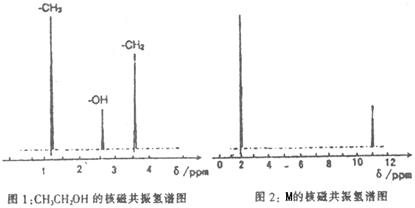
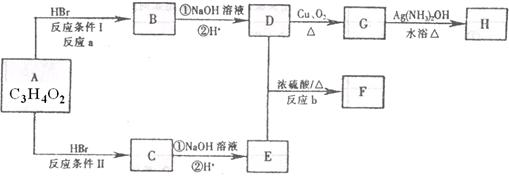
��֪����A��M����ͼ��ʾ�Ĺ�ϵ���ҳ����£�A��MΪ��ɫ��dz��ɫ���壬GΪ��ɫ���壬B��KΪ���嵥�ʣ�E��F��HΪҺ�壻D������ʹ�����ʯ��ˮ������ɫ������D��K��ˮ��Һ������Ư���ԣ�������Ϻ�һ������Ư���ԣ����F��M��Ԫ�ع������֣���F��M��ֻ������Ԫ����ɣ�����Ԫ�ص�ԭ�Ӹ���֮�Ⱦ�Ϊ1:1��
�Իش���������:
(1)M�ĵ���ʽΪ:_______________��M��������������֮��Ϊ:_______________��
(2)G�ڷ�Ӧ�٣����зֱ���_______________����_______________����
(3)д�����з�Ӧ�Ļ�ѧ����ʽ:
A+B��C+D:______________________________��
D+M��L:________________________________��
F��G�������B��E:______________________________��
(4)д�����з�Ӧ�����ӷ���ʽ:
K+E+D��I+H:______________________________��
I+G��K+E+J:______________________________��
![]()
(2)������
��3��4FeS2+11O2![]() 2Fe2O3+8SO2
2Fe2O3+8SO2
SO2+Na2O2![]() Na2SO4
Na2SO4
2 H2O2![]() 2H2O+O2��
2H2O+O2��
(4)SO2+Cl2+2H2O![]() 4H-+
4H-+![]() +2Cl-
+2Cl-
MnO2+4H++2Cl-(Ũ)![]() Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O

��ϰ��ϵ�д�
�����Ŀ