��Ŀ����
�ϳɰ���ҵ�ĺ��ķ�Ӧ�ǣ�
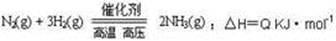
�����仯����ͼ���ش��������⣺
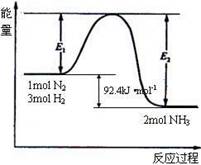
��1���ڷ�Ӧ��ϵ�м����������Ӧ��������E1��E2�ı仯�ǣ�E1�������� ��E2�������������� �����������С���������䡱��
��2����500�桢2��107Pa�ʹ�����������һ�ܱ������г���0.5mol N2��1.5mol H2����ַ�Ӧ�ų����������������� ���<������>����=����46.2kJ��
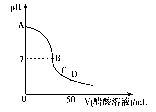
��3�����ڸ÷�Ӧ������˵���У���ȷ�������������������������� ��
A����H>0����S>0������ B����H>0����S< 0���� C����H<0����S>0������ D����H<0����S<0��4����һ������N2��g����H2��g������ 1L�ܱ������У���500�桢2��107Pa�´ﵽƽ�⣬���N2Ϊ0.10mol��H2Ϊ0.30mol��NH3Ϊ0.10mol������������´ﵽƽ��ʱH2ת��ΪNH3��ת������������������������ ���������¶ȣ�Kֵ�仯���������� �����������С�����䡱����
��5����������4����Ӧ�������ܱ������У�����ߺϳɰ���H2��ת���ʣ����д�ʩ���е������������������� ������ĸ����
A���������а�ԭ�����ٳ���ԭ�������������� B�����������ٳ����������
C���ı䷴Ӧ�Ĵ������������������������� D���������
�����仯����ͼ���ش��������⣺

��1���ڷ�Ӧ��ϵ�м����������Ӧ��������E1��E2�ı仯�ǣ�E1�������� ��E2�������������� �����������С���������䡱��
��2����500�桢2��107Pa�ʹ�����������һ�ܱ������г���0.5mol N2��1.5mol H2����ַ�Ӧ�ų����������������� ���<������>����=����46.2kJ��
��3�����ڸ÷�Ӧ������˵���У���ȷ�������������������������� ��
A����H>0����S>0������ B����H>0����S< 0���� C����H<0����S>0������ D����H<0����S<0��4����һ������N2��g����H2��g������ 1L�ܱ������У���500�桢2��107Pa�´ﵽƽ�⣬���N2Ϊ0.10mol��H2Ϊ0.30mol��NH3Ϊ0.10mol������������´ﵽƽ��ʱH2ת��ΪNH3��ת������������������������ ���������¶ȣ�Kֵ�仯���������� �����������С�����䡱����
��5����������4����Ӧ�������ܱ������У�����ߺϳɰ���H2��ת���ʣ����д�ʩ���е������������������� ������ĸ����
A���������а�ԭ�����ٳ���ԭ�������������� B�����������ٳ����������
C���ı䷴Ӧ�Ĵ������������������������� D���������
��1����С�� ��С
��2��<
��3��D
��4��33.3%����С
��5��A��D
��2��<
��3��D
��4��33.3%����С
��5��A��D
��1������������ܽ��ͷ�Ӧ����Ļ�ܣ�����Ӱ�췴Ӧ�ȣ�����E1��E2�ľ���С��
��2���÷�ӦΪ���淴Ӧ����Ӧ�ﲻ����ȫת��Ϊ��������ԣ�����0.5mol N2��1.5mol H2����ַ�Ӧ�ų�������С��46.2kJ��
��3���÷�ӦΪ���ȷ�Ӧ�����ԡ�H<0���ָ÷�ӦΪ�����������ķ�Ӧ�����ԡ�S<0��
��4��33.3%���÷�ӦΪ���ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ�����K��С
��5����ߺϳɰ���H2��ת���ʼ�ʹƽ��������Ӧ�����ƶ������ԣ���ѡ���У�A��D
��2���÷�ӦΪ���淴Ӧ����Ӧ�ﲻ����ȫת��Ϊ��������ԣ�����0.5mol N2��1.5mol H2����ַ�Ӧ�ų�������С��46.2kJ��
��3���÷�ӦΪ���ȷ�Ӧ�����ԡ�H<0���ָ÷�ӦΪ�����������ķ�Ӧ�����ԡ�S<0��
��4��33.3%���÷�ӦΪ���ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ�����K��С
��5����ߺϳɰ���H2��ת���ʼ�ʹƽ��������Ӧ�����ƶ������ԣ���ѡ���У�A��D

��ϰ��ϵ�д�
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ
 CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���ٴ˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ ��
CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���ٴ˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ �� CO (g)+2H2O (g) +519KJ����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��
CO (g)+2H2O (g) +519KJ����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��
 2SO3(g)����H < 0 �����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯
2SO3(g)����H < 0 �����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯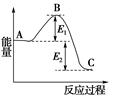
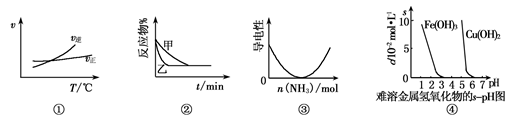
 2AB3(g)�Ħ�H>0
2AB3(g)�Ħ�H>0
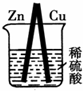
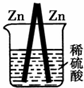
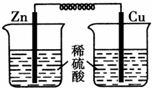
 ͼ��
ͼ��