ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩœ¬±μ «‘ΣΥΊ÷ήΤΎ±μ“Μ≤ΩΖ÷Θ§Ν–≥ωΝΥ °Ηω‘ΣΥΊ‘Ύ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο:
Ήε ÷ήΤΎ | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
2 | Δό | ΔΏ | ||||||
3 | ΔΌ | Δέ | Δί | Δύ | Δβ | |||
4 | ΔΎ | Δή | Δα |
«κ”ΟΜ·―ß”Ο”οΜΊ¥πœ¬Ν–Έ Χβ
Θ®1Θ©‘ΎΔέΓΪΔΏ‘ΣΥΊ÷–Θ§‘≠Ή”ΑκΨΕΉν¥σΒΡ «_______________Θ®Χν‘ΣΥΊΖϊΚ≈Θ©ΘΜ
Θ®2Θ©ΔΌΓΪΔβ÷–‘ΣΥΊΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·Έο÷–Υα–‘Ήν«ΩΒΡ «_______________Θ®ΧνΈο÷ Μ·―ß ΫΘ©Θ§≥ ΝΫ–‘ΒΡ«β―θΜ·Έο «_____________Θ®ΧνΈο÷ Μ·―ß ΫΘ©ΘΜ
Θ®3Θ©ΔΏ‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·Έο”κΤδ«βΜ·ΈοΡή…ζ≥…―ΈMΘ§/span>M÷–Κ§”–ΒΡΜ·―ßΦϋάύ–Ά”–________________________ΘΜ
Θ®4Θ©”ΟΒγΉ” Ϋ±μ Ψ‘ΣΥΊΔέ”κΔύ–Έ≥…Μ·ΚœΈοΒΡΙΐ≥Χ_________________________________ΓΘ
Θ®5Θ©–¥≥ωΚ§”–32ΗωΒγΉ”ΒΡ‘ΣΥΊΔόΒΡ«βΜ·ΈοΒΡΖ÷Ή” ΫΘΚ_____________________________ΓΘ
Θ®6Θ©–¥≥ωΙΛ“Β“±ΝΕΔίΒΡΜ·―ßΖΫ≥Χ ΫΘΚ____________________________________
Θ®7Θ©–¥≥ωΫΪΔα‘ΣΥΊΒΞ÷ ¥”ΚΘΥ°÷–Θ®άκΉ”–Έ Ϋ¥φ‘ΎΘ©Χα»ΓΥυ…φΦΑΒΫΒΡ»ΐΗω≤Ϋ÷ηΒΡάκΉ”ΖΫ≥Χ ΫΘ§ΒΎ“Μ≤ΫΘΚCl2+2Br-ΘΫ2Cl-+Br2 ΒΎΕΰ≤ΫΘΚ____________________________ ΘΜ ΒΎ»ΐ≤ΫCl2+2Br-ΘΫ2Cl-+Br2
ΓΨ¥πΑΗΓΩΘ®1Θ©Ca
Θ®2Θ©HClO4ΓΔ Al(OH)3
Θ®3Θ©άκΉ”ΦϋΓΔΘ®ΦΪ–‘Θ©Ι≤ΦέΦϋ
Θ®4Θ©![]()
Θ®5Θ©C4H8
Θ®6Θ©2Al2O3![]() 4Al +3O2Γϋ ±υΨß ·
4Al +3O2Γϋ ±υΨß ·
Θ®7Θ©Br2+ SO2+2H2OΘΫ4H++ SO42-+ 2Br-
ΓΨΫβΈωΓΩ
‘ΧβΔΌΓΪΔβΗς‘ΣΥΊΖ÷±πΈΣNaΓΔKΓΔMgΓΔCaΓΔAlΓΔCΓΔNΓΔClΓΔBrΚΆArΓΘΘ®1Θ©ΒγΉ”≤ψ ΐΉνΕύΒΡ‘ΣΥΊΘ§Τδ‘≠Ή”ΑκΨΕΉν¥σΘΜΘ®2Θ©‘ΣΥΊΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·ΈοΥα–‘Ήν«ΩΘ§‘ρΗΟ‘ΣΥΊΒΡΖ«Ϋπ τ–‘Ήν«ΩΘΜΘ®3Θ©MΈο÷ ΈΣNH4NO3Θ§άκΉ”Μ·ΚœΈοΘ§Κ§”–άκΉ”ΦϋΚΆΙ≤ΦέΦϋΘΜΘ®4Θ©‘ΣΥΊΔέ”κΔύ–Έ≥…Μ·ΚœΈο¬»Μ·ΟΨΘ§άκΉ”Μ·ΚœΈοΘ§Ι ”ΟΦΐΆΖ±μ ΨΒγΉ”ΒΡΉΣ“ΤΘΜΘ®5Θ©‘ΣΥΊΔόΒΡ«βΜ·ΈοΈΣCxHyΘ§ΒγΉ”ΈΣ12x+y=32Θ§xΓΔy÷°ΦδΒΡΙΊœΒΜΙ”ΠΖϊΚœΆιΧΰΜρœ©ΧΰΜρ»≤ΧΰΒΡΆ® ΫΘΜΘ®6Θ©Al «ΜνΤΟΫπ τΘ§ΙΛ“Β“±ΝΕ–η”ΟΒγΫβΖ®ΓΘ

ΓΨΧβΡΩΓΩΔώΘ°ZrO2≥Θ”ΟΉςΧ’¥…≤ΡΝœΘ§Ω…”…ο·”Δ…Α(÷ς“Σ≥…Ζ÷ΈΣZrSiO4Θ§“≤Ω…±μ ΨΈΣZrO2ΓΛSiO2Θ§ΜΙΚ§…ΌΝΩFe2O3ΓΔAl2O3ΓΔSiO2Β»‘”÷ )Ά®Ιΐ»γœ¬ΖΫΖ®÷Τ»ΓΘΚ
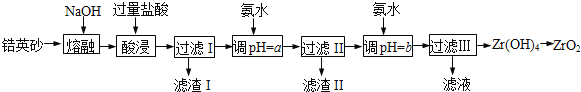
“―÷ΣΘΚΔΌZrO2Ρή”κ…’ΦνΖ¥”Π…ζ≥…Ω…»ή”ΎΥ°ΒΡNa2ZrO3Θ§Na2ZrO3”κΥαΖ¥”Π…ζ≥…ZrO2+ΓΘ
ΔΎ≤ΩΖ÷Ϋπ τάκΉ”‘Ύ Β―ιΧθΦΰœ¬ΩΣ Φ≥ΝΒμΚΆΆξ»Ϊ≥ΝΒμΒΡpH»γœ¬±μΓΘ
Ϋπ τάκΉ” | Fe3+ | Al3+ | ZrO2+ |
ΩΣ Φ≥ΝΒμ ±pH | 1.9 | 3.3 | 6.2 |
≥ΝΒμΆξ»Ϊ ±pH | 3.2 | 5.2 | 8.0 |
Θ®1Θ©»έ»Ύ ±ZrSiO4ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___________________________________________Θ§¬Υ‘ϋIΒΡΜ·―ß ΫΈΣ___________________
Θ®2Θ©ΈΣ Ι¬Υ“ΚIΒΡ‘”÷ άκΉ”≥ΝΒμΆξ»ΪΘ§–η”ΟΑ±Υ°ΒςpH=aΘ§‘ρaΒΡΖΕΈß «____________________ΘΜΦΧ–χΦ”Α±Υ°÷ΝpH=b ±Θ§ΥυΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ__________________________________________
Θ®3Θ©œρΙΐ¬ΥIIIΥυΒΟ¬Υ“Κ÷–Φ”»κCaCO3ΖέΡ©≤ΔΦ”»»Θ§ΒΟΒΫΝΫ÷÷ΤχΧεΓΘΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚ________________________________________
Θ®4Θ©ΈΣΒΟΒΫ¥ΩΨΜΒΡZrO2Θ§Zr(OH)4–η“Σœ¥Β”Θ§Φλ―ιZr(OH)4 «Ζώœ¥Β”Η…ΨΜΒΡΖΫΖ® «ΘΚ____________________________
ΔρΘ°ΒΣΜ·ΙηΘ®Si3N4Θ© «“Μ÷÷–¬–ΆΧ’¥…≤ΡΝœΘ§ΥϋΩ…”Ο ·”Δ”κΫΙΧΩ‘ΎΗΏΈ¬ΒΡΒΣΤχΝς÷–Ζ¥”Π÷ΤΒΟΘΚ___SiO2 + C + N2![]() Si3N4 + CO
Si3N4 + CO
ΗυΨίΧβ“βΆξ≥…œ¬Ν–ΗςΧβΘΚ
Θ®1Θ©≈δΤΫ…œ ωΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΓΘ__________
Θ®2Θ©ΈΣΝΥ±Θ÷Λ ·”ΔΚΆΫΙΧΩΨΓΩ…ΡήΒΡΉΣΜ·Θ§ΒΣΤχ“Σ Β±ΙΐΝΩΓΘΡ≥¥ΈΖ¥”Π”ΟΝΥ30 molΒΣΤχΘ§Ζ¥”Π…ζ≥…ΝΥ5 mol“Μ―θΜ·ΧΦΘ§‘ρ¥Υ ±ΜλΚœΤχΧεΒΡΤΫΨυΡΠΕϊ÷ ΝΩ «______________ΓΘ
Θ®3Θ©ΒΣΜ·ΙηΧ’¥…ΒΡΜζ–Β«ΩΕ»ΗΏΘ§”≤Ε»Ϋ”Ϋϋ”ΎΗ’”ώΘ®A12O3Θ©Θ§»»Έ»Ε®–‘ΚΟΘ§Μ·―ß–‘÷ Έ»Ε®ΓΘ“‘œ¬”ΟΆΨ’ΐ»ΖΒΡ «__________Θ®Χν–ρΚ≈Θ©
AΘ°Ω…“‘‘Ύ“±ΫπΙΛ“Β…œ÷Τ≥…έαέωΓΔ¬ΝΒγΫβ≤έ≥ΡάοΒ»…η±Η
BΘ°‘ΎΒγΉ”ΙΛ“Β…œ÷Τ≥…ΡΆΗΏΈ¬ΒΡΒγΒΡΝΦΒΦΧε
CΘ°―–ΖΔΒΣΜ·ΙηΒΡ»ΪΧ’ΖΔΕ·ΜζΧφ¥ζΆ§άύ–ΆΫπ τΖΔΕ·Μζ
DΘ°ΒΣΜ·ΙηΧ’¥…ΒΡΩΣΖΔ ήΒΫΉ ‘¥ΒΡœό÷ΤΘ§ΟΜ”–ΖΔ’Ι«ΑΆΨ