��Ŀ����
4�ֶ�����Ԫ��A��B��C��D�������Ϣ���£���Ϣ��ԭ�Ӱ뾶��С��A��B��C��D��Ϣ��4��Ԫ�ص�ԭ��֮���γɵļס��Һͱ�3�����ʵIJ�����Ϣ���£�
�����������Ϣ�ش��������⣮
��1��Ԫ��B�����ڱ��е�λ��
 ��
��
��2��Ԫ��E��F��Ԫ��A����ͬһ���ڣ�E���ʵĻ�ԭ����ǿ��������ܷ�Ӧ���ɻ�����X����Ԫ��F��ijЩ�ο���Ϊ��ˮ������Ԫ��F��C��ɵĻ�����Y����Ϊ�ͻ���ϣ�
��д��Ԫ��A�ĵ������Ӧ�Ļ�ѧ����ʽ��
��д��������Y�ܽ���X��Һ�����ӷ���ʽ��
| �� | �� | �� | |
| ���� ����ģ�� |
 |
 |
 |
| �������� | ��������������ʣ���������������Ҫ����֮һ��Լռ����������2/3�� | ��ɫ������ζ������ȼ���dz�����һ�ֻ�����Դ�� | ��ǿ�����Ե����ᣬ������������ɱ���� |
��1��Ԫ��B�����ڱ��е�λ��
��2����IVA��
��2����IVA��
�������ӵĵ���ʽ

��2��Ԫ��E��F��Ԫ��A����ͬһ���ڣ�E���ʵĻ�ԭ����ǿ��������ܷ�Ӧ���ɻ�����X����Ԫ��F��ijЩ�ο���Ϊ��ˮ������Ԫ��F��C��ɵĻ�����Y����Ϊ�ͻ���ϣ�
��д��Ԫ��A�ĵ������Ӧ�Ļ�ѧ����ʽ��
Cl2+H2O=HCl+HClO
Cl2+H2O=HCl+HClO
����д��������Y�ܽ���X��Һ�����ӷ���ʽ��
Al2O3+2OH-=2AlO2-+H2O
Al2O3+2OH-=2AlO2-+H2O
�����������ǵ�������������ʣ���������������Ҫ����֮һ��Լռ����������
�����ӦΪH2O�����Ԫ��ΪH��O��
������ɫ������ζ������ȼ���dz�����һ�ֻ�����Դ���ɱ���ģ�Ϳ�֪��ΪCH4����ϳ�Ԫ��ΪC��H��
������ǿ�����Ե����ᣬ������������ɱ�����ɱ���ģ�Ϳ�֪��ΪHClO������Ԫ��ΪH��Cl��O��
ԭ�Ӱ뾶Cl��C��O��H�����ԭ�Ӱ뾶��С��A��B��C��D������AΪCl��BΪC��CΪO��DΪH��
Ԫ��E��F��Ԫ��A����Cl������ͬһ���ڣ�E���ʵĻ�ԭ����ǿ��ӦΪNa������ף���H2O���ܷ�Ӧ���ɻ�����X��ӦΪNaOH����Ԫ��F��ijЩ�ο���Ϊ��ˮ������Ԫ��F��C��ɵĻ�����Y����Ϊ�ͻ���ϣ�ӦΪAl2O3����FΪAl��
��϶�Ӧԭ�ӵĽṹ�Լ����ʡ�����������ʽ����⣮
| 2 |
| 3 |
������ɫ������ζ������ȼ���dz�����һ�ֻ�����Դ���ɱ���ģ�Ϳ�֪��ΪCH4����ϳ�Ԫ��ΪC��H��
������ǿ�����Ե����ᣬ������������ɱ�����ɱ���ģ�Ϳ�֪��ΪHClO������Ԫ��ΪH��Cl��O��
ԭ�Ӱ뾶Cl��C��O��H�����ԭ�Ӱ뾶��С��A��B��C��D������AΪCl��BΪC��CΪO��DΪH��
Ԫ��E��F��Ԫ��A����Cl������ͬһ���ڣ�E���ʵĻ�ԭ����ǿ��ӦΪNa������ף���H2O���ܷ�Ӧ���ɻ�����X��ӦΪNaOH����Ԫ��F��ijЩ�ο���Ϊ��ˮ������Ԫ��F��C��ɵĻ�����Y����Ϊ�ͻ���ϣ�ӦΪAl2O3����FΪAl��
��϶�Ӧԭ�ӵĽṹ�Լ����ʡ�����������ʽ����⣮
����⣺���ǵ�������������ʣ���������������Ҫ����֮һ��Լռ����������
�����ӦΪH2O�����Ԫ��ΪH��O��
������ɫ������ζ������ȼ���dz�����һ�ֻ�����Դ���ɱ���ģ�Ϳ�֪��ΪCH4����ϳ�Ԫ��ΪC��H��
������ǿ�����Ե����ᣬ������������ɱ�����ɱ���ģ�Ϳ�֪��ΪHClO������Ԫ��ΪH��Cl��O��
ԭ�Ӱ뾶Cl��C��O��H�����ԭ�Ӱ뾶��С��A��B��C��D������AΪCl��BΪC��CΪO��DΪH����
��1��BΪC��ԭ������Ϊ6��ԭ�Ӻ�����2�����Ӳ㣬����������Ϊ4����λ�����ڱ���2����IVA�壬
��ΪHClO������ʽΪ ��
��
�ʴ�Ϊ����2����IVA�壻 ��
��
��2��Ԫ��E��F��Ԫ��A����Cl������ͬһ���ڣ�E���ʵĻ�ԭ����ǿ��ӦΪNa������ף���H2O���ܷ�Ӧ���ɻ�����X��ӦΪNaOH����Ԫ��F��ijЩ�ο���Ϊ��ˮ������Ԫ��F��C��ɵĻ�����Y����Ϊ�ͻ���ϣ�ӦΪAl2O3����FΪAl��
��Cl2��H2O��Ӧ�Ļ�ѧ����ʽΪCl2+H2O=HCl+HClO��
�ʴ�Ϊ��Cl2+H2O=HCl+HClO��
�ڻ�����YΪAl2O3��XΪNaOH�����߷�Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
| 2 |
| 3 |
������ɫ������ζ������ȼ���dz�����һ�ֻ�����Դ���ɱ���ģ�Ϳ�֪��ΪCH4����ϳ�Ԫ��ΪC��H��
������ǿ�����Ե����ᣬ������������ɱ�����ɱ���ģ�Ϳ�֪��ΪHClO������Ԫ��ΪH��Cl��O��
ԭ�Ӱ뾶Cl��C��O��H�����ԭ�Ӱ뾶��С��A��B��C��D������AΪCl��BΪC��CΪO��DΪH����
��1��BΪC��ԭ������Ϊ6��ԭ�Ӻ�����2�����Ӳ㣬����������Ϊ4����λ�����ڱ���2����IVA�壬
��ΪHClO������ʽΪ
 ��
���ʴ�Ϊ����2����IVA�壻
 ��
����2��Ԫ��E��F��Ԫ��A����Cl������ͬһ���ڣ�E���ʵĻ�ԭ����ǿ��ӦΪNa������ף���H2O���ܷ�Ӧ���ɻ�����X��ӦΪNaOH����Ԫ��F��ijЩ�ο���Ϊ��ˮ������Ԫ��F��C��ɵĻ�����Y����Ϊ�ͻ���ϣ�ӦΪAl2O3����FΪAl��
��Cl2��H2O��Ӧ�Ļ�ѧ����ʽΪCl2+H2O=HCl+HClO��
�ʴ�Ϊ��Cl2+H2O=HCl+HClO��
�ڻ�����YΪAl2O3��XΪNaOH�����߷�Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
���������⿼��������ƶ��Լ�Ԫ�ص��ƶϣ���Ŀ�Ѷ��еȣ������ע����ճ������ʵı���ģ�ͣ���ѧϰ��ע�����������ʵ����ʣ�

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ
4�ֶ�����Ԫ��A��B��C��D�������Ϣ���£���Ϣ��ԭ�Ӱ뾶��С��A��B��C��D��Ϣ��4��Ԫ�ص�ԭ��֮���γɵļס��Һͱ�3�����ʵIJ�����Ϣ���£�
| �� | �� | �� | |
| ���� ����ģ�� |  |  |  |
| �������� | ��������������ʣ���������������Ҫ����֮һ��Լռ����������2/3�� | ��ɫ������ζ������ȼ���dz�����һ�ֻ�����Դ�� | ��ǿ�����Ե����ᣬ������������ɱ���� |
��1��Ԫ��B�����ڱ��е�λ��______�������ӵĵ���ʽ______��
��2��Ԫ��E��F��Ԫ��A����ͬһ���ڣ�E���ʵĻ�ԭ����ǿ��������ܷ�Ӧ���ɻ�����X����Ԫ��F��ijЩ�ο���Ϊ��ˮ������Ԫ��F��C��ɵĻ�����Y����Ϊ�ͻ���ϣ�
��д��Ԫ��A�ĵ������Ӧ�Ļ�ѧ����ʽ��______��
��д��������Y�ܽ���X��Һ�����ӷ���ʽ��______��
4�ֶ�����Ԫ��A��B��C��D�������Ϣ���£���Ϣ��ԭ�Ӱ뾶��С��A��B��C��D��Ϣ��4��Ԫ�ص�ԭ��֮���γɵļס��Һͱ�3�����ʵIJ�����Ϣ���£�
�����������Ϣ�ش��������⣮
��1��Ԫ��B�����ڱ��е�λ��______�������ӵĵ���ʽ______��
��2��Ԫ��E��F��Ԫ��A����ͬһ���ڣ�E���ʵĻ�ԭ����ǿ��������ܷ�Ӧ���ɻ�����X����Ԫ��F��ijЩ�ο���Ϊ��ˮ������Ԫ��F��C��ɵĻ�����Y����Ϊ�ͻ���ϣ�
��д��Ԫ��A�ĵ������Ӧ�Ļ�ѧ����ʽ��______��
��д��������Y�ܽ���X��Һ�����ӷ���ʽ��______��
| �� | �� | �� | |
| ���� ����ģ�� | 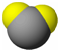 |  |  |
| �������� | ��������������ʣ���������������Ҫ����֮һ��Լռ����������2/3�� | ��ɫ������ζ������ȼ���dz�����һ�ֻ�����Դ�� | ��ǿ�����Ե����ᣬ������������ɱ���� |
��1��Ԫ��B�����ڱ��е�λ��______�������ӵĵ���ʽ______��
��2��Ԫ��E��F��Ԫ��A����ͬһ���ڣ�E���ʵĻ�ԭ����ǿ��������ܷ�Ӧ���ɻ�����X����Ԫ��F��ijЩ�ο���Ϊ��ˮ������Ԫ��F��C��ɵĻ�����Y����Ϊ�ͻ���ϣ�
��д��Ԫ��A�ĵ������Ӧ�Ļ�ѧ����ʽ��______��
��д��������Y�ܽ���X��Һ�����ӷ���ʽ��______��