��Ŀ����
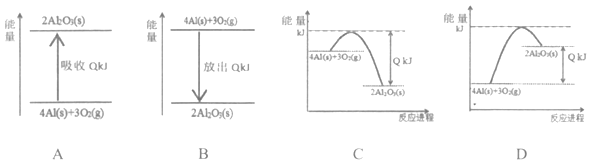
����Ŀ����(Sb)���仯�����ڹ�ҵ����������;���Ի����(��Ҫ�ɷ�ΪSb2S3��������PbS��As2S3��CuO��SiO2��)Ϊԭ���Ʊ�������Ĺ���������ͼ��ʾ��

��֪���ٽ���Һ�г������������SbCl5֮�⣬������SbCl3��PbCl2��AsCl3��CuCl2�ȣ�
�ڳ����£�Ksp(CuS)��1.27��10��36��Ksp(PbS)��9.04��10��29��
����Һ������Ũ����1.0��10��5mol��L��1ʱ����Ϊ�����ӳ�����ȫ��
(1)����1�г���S֮�⣬����________ (�ѧʽ)��
(2)��������ʱ��Sb2S3������Ӧ�Ļ�ѧ����ʽΪ___________________________________��
(3)����ԭ��ʱ����Sb��ԭ������Ϊ________(�ѧʽ)��
(4)�����£�����ͭ��Ǧ��ʱ��Cu2����Pb2����������ȫ����ʱ��Һ�е�c(S2��)������_____������Na2SҲ���˹��࣬��ԭ��Ϊ_________________________________��
(5)��������ʱ��H3PO3���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ________________________________��
(6)�������ʱ����������SbԪ���뱻��ԭ��SbԪ�ص�����֮��Ϊ________________��
���𰸡�SiO2 Sb2S3��3SbCl5===5SbCl3��3S SbCl5 9.04��10��24 mol��L��1 ����H2S����Ⱦ������(������Sb2S3) 2AsCl3��3Na3PO2��3HCl��3H2O===2As����3H3PO3��9NaCl 3��2
��������
(1) SiO2���������
(2)��������1��S��˵����������ʱSb2S3�е�SԪ�ر�SbCl5����ΪS����������ͼ��֪��SbCl5����ԭΪSbCl3��
(3)����ԭ��ʱ������������Sb��ԭ��������ʱ����Ĺ���SbCl5��
(4) ����Ksp(PbS)��9.04��10��29����Cu2����Pb2����������ȫʱ��Һ�е�c(S2��)����Сֵ��
(5)�����顱ʱNa3PO2��AsCl3����������Ӧ��Ӧ����As��H3PO3��
(6)����⡱ʱSbCl3������������ΪSbCl5��SbCl3����������ԭΪSb��
(1) SiO2���������ᣬ��������1�г���S֮�⣬����SiO2��
(2) ��������ʱSb2S3�е�SԪ�ر�SbCl5����ΪS��SbCl5����ԭΪSbCl3����Ӧ��ѧ����ʽ��Sb2S3��3SbCl5=5SbCl3��3S��
(3)����ԭ��ʱ������������Sb��ԭ��������ʱ����Ĺ���SbCl5�����Ա�Sb��ԭ������ΪSbCl5��
(4)Pb2����ȫ����ʱ��c(Pb2��)��1.0��10��5mol��L��1��Ksp(PbS)��9.04��10��29��������Һ�е�c(S2��) ![]()
![]() 9.04��10��24 mol��L��1������Na2S���࣬������H2S����Ⱦ�����塣
9.04��10��24 mol��L��1������Na2S���࣬������H2S����Ⱦ�����塣
(5)�����顱ʱNa3PO2��AsCl3����������Ӧ��Ӧ����As��H3PO3����Ӧ��ѧ����ʽΪ2AsCl3��3Na3PO2��3HCl��3H2O=2As����3H3PO3��9NaCl��
(6)����⡱ʱSbCl3������������ΪSbCl5��SbCl3����������ԭΪSb�����ݵ�ʧ�����غ㣬��������SbԪ���뱻��ԭ��SbԪ�ص�����֮��Ϊ3:2��

����Ŀ�������¢١�������װ��50mL��������Ͳ���ֱ��ȡ10mLH2O��10mL15%��NaOH��Һ����ͼ��ʾ����������ã�������Ͳ��������Һ�����������

A | B | C | D | |
��Ͳ�� | ����ɫ | ��Ư���� | ������ | ��Cl2���� |
��Ͳ�� | ����ɫ | ��Ư���� | �ʼ��� | ��Cl2���� |
A. AB. BC. CD. D