��Ŀ����
��15�֣�����ͭ�ڻ�����ũҵ�����кܹ㷺���ô���ij��ѧ��ȤС��������ϣ������ֲ�ͬ��ԭ����ȡ����ͭ��
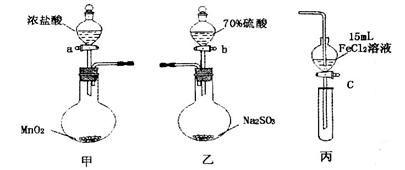
��ʽһ��һ�ֺ�ͭ�Ŀ�ʯ�����ȸʯ��ۣ�����ͭ��̬ΪCuCO3��Cu(OH)2��CuSiO3��2H2O������SiO2��FeCO3��Fe2O3��Al2O3�����ʣ��������ֿ�ʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��

��ش��������⣺
����ɲ������ϡ������CuSiO3��2H2O������Ӧ�Ļ�ѧ����ʽ
CuSiO3��2H2O+H2SO4=CuSO4 +________+H2O��
�Ʋ���ڵ�����ҺpHѡ�õ�����Լ���__________________
A. CuO B. MgO C. FeCO3 D NH3��H2O
���й��������↑ʼ��������ȫ������pH���±���
���ϱ���֪������ҺpH=4ʱ��������ȫ��ȥ��������________��
����ҺBͨ������Ũ���������Ũ��Ϊԭ����һ�룩����ȴ�ᾧ���Եõ�CuSO4��5H2O���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�Al2(SO4)3 ������Һ��C(Al3+)=2.25mol��L-1��Ksp[Al(OH)3]=3.2��10-34) ________�����ȷ��������
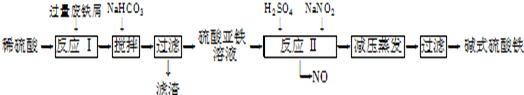
��ʽ�����Ի�ͭ��Ϊԭ�ϣ���ȡ����ͭ������ͭ�Ĺ���������ʾ��
��.����ͭ����Ҫ�ɷ�ΪCuFeS2����������CaO��MgO��Al2O3������
��.��������װ�ý��е绯ѧ����ʵ�飬����ѡ��ͭ��ۼ�����������������ٽ��裬ʹ����ܽ⡣��������ͨ������������������������

��.һ��ʱ���ȡ��������Һ�������м����л���ȡ����RH��������Ӧ��
2RH���л��ࣩ+ Cu2+��ˮ�ࣩ R2Cu���л��ࣩ+ 2H����ˮ�ࣩ
R2Cu���л��ࣩ+ 2H����ˮ�ࣩ
������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+����������
��.�������ͭ��Һ�Ƶý���ͭ��
��5����ͭ��ۼ��������������ἰ��������Ҫ�������·�Ӧ��
CuFeS2 + 4H�� = Cu2+ + Fe2+ + 2H2S 2Fe3+ + H2S = 2Fe2+ + S��+ 2H��
�������У�������Fe3+��Ũ�Ȼ������ֲ��䣬ԭ����____________________���õ缫��Ӧʽ��ʾ����
��6����������л����м���һ��Ũ�ȵ����ᣬCu2+����������ԭ����_____________ ��
��7��������������0.1mol CuSO4��Һ������ͭ3.2 g����ʱ��Һ������Ũ���ɴ�С��˳���� ____ ��
��ʽһ��һ�ֺ�ͭ�Ŀ�ʯ�����ȸʯ��ۣ�����ͭ��̬ΪCuCO3��Cu(OH)2��CuSiO3��2H2O������SiO2��FeCO3��Fe2O3��Al2O3�����ʣ��������ֿ�ʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��

��ش��������⣺
����ɲ������ϡ������CuSiO3��2H2O������Ӧ�Ļ�ѧ����ʽ
CuSiO3��2H2O+H2SO4=CuSO4 +________+H2O��
�Ʋ���ڵ�����ҺpHѡ�õ�����Լ���__________________
A. CuO B. MgO C. FeCO3 D NH3��H2O
���й��������↑ʼ��������ȫ������pH���±���
| �������� | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 |
����ҺBͨ������Ũ���������Ũ��Ϊԭ����һ�룩����ȴ�ᾧ���Եõ�CuSO4��5H2O���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�Al2(SO4)3 ������Һ��C(Al3+)=2.25mol��L-1��Ksp[Al(OH)3]=3.2��10-34) ________�����ȷ��������
��ʽ�����Ի�ͭ��Ϊԭ�ϣ���ȡ����ͭ������ͭ�Ĺ���������ʾ��
��.����ͭ����Ҫ�ɷ�ΪCuFeS2����������CaO��MgO��Al2O3������
��.��������װ�ý��е绯ѧ����ʵ�飬����ѡ��ͭ��ۼ�����������������ٽ��裬ʹ����ܽ⡣��������ͨ������������������������

��.һ��ʱ���ȡ��������Һ�������м����л���ȡ����RH��������Ӧ��
2RH���л��ࣩ+ Cu2+��ˮ�ࣩ
 R2Cu���л��ࣩ+ 2H����ˮ�ࣩ
R2Cu���л��ࣩ+ 2H����ˮ�ࣩ������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+����������
��.�������ͭ��Һ�Ƶý���ͭ��
��5����ͭ��ۼ��������������ἰ��������Ҫ�������·�Ӧ��
CuFeS2 + 4H�� = Cu2+ + Fe2+ + 2H2S 2Fe3+ + H2S = 2Fe2+ + S��+ 2H��
�������У�������Fe3+��Ũ�Ȼ������ֲ��䣬ԭ����____________________���õ缫��Ӧʽ��ʾ����
��6����������л����м���һ��Ũ�ȵ����ᣬCu2+����������ԭ����_____________ ��
��7��������������0.1mol CuSO4��Һ������ͭ3.2 g����ʱ��Һ������Ũ���ɴ�С��˳���� ____ ��
��15�֣���1��H4SiO4��2�֣�
��2��A ��2�֣�
��3��Al3+��2�֣�
��4������3�֣�
��5��Fe2+- e��=Fe3+��2�֣�
��6������H+Ũ�ȣ�ʹƽ��2RH(�л���) + Cu2+(ˮ��) R2Cu(�л���)+ 2H��(ˮ��)�����ƶ���Cu2+����ˮ��������� ��2�֣�
R2Cu(�л���)+ 2H��(ˮ��)�����ƶ���Cu2+����ˮ��������� ��2�֣�
��7�� c(H+)>c(SO42-)>c(Cu2+)>c(OH-)��2�֣�
��2��A ��2�֣�
��3��Al3+��2�֣�
��4������3�֣�
��5��Fe2+- e��=Fe3+��2�֣�
��6������H+Ũ�ȣ�ʹƽ��2RH(�л���) + Cu2+(ˮ��)
 R2Cu(�л���)+ 2H��(ˮ��)�����ƶ���Cu2+����ˮ��������� ��2�֣�
R2Cu(�л���)+ 2H��(ˮ��)�����ƶ���Cu2+����ˮ��������� ��2�֣���7�� c(H+)>c(SO42-)>c(Cu2+)>c(OH-)��2�֣�
�����������1�����������غ㶨�ɣ��հ״���������H4SiO4��
��2������ڵ�����ҺpH��Ŀ���dz�ȥ�����ӵ����ʣ����Ե���pH���������µ����ʣ����Դ�ѡA����3��pH=4ʱ��������ȫ��������Al3+��ȫ������pH=5.2�����Ե���ҺpH=4ʱ��������ȫ��ȥ������ �� Al3+��
��4����PH=4ʱ����Һ�е����������ӵ�Ũ��Ϊ��1��10-10mol/L����ʱ���������ѳ���������Һ��������ŨΪ��3.2��10-34/(1��10-10)3=3.2��10-4mol/L��Ũ����c��Al3+��=6.4��10-4mol/L����2.25mol/L�����Բ������������������������Ը�ͬѧ�Ĺ۵����
��5����������������Ӧ����ͭ��������ἰ��������Ӧ���ɵ��������ӱ��������������ӣ������������������ӵ�Ũ�Ȼ������䣬�缫��ӦʽΪFe2+- e��=Fe3+��
��6����������л����м���һ��Ũ�ȵ����ᣬʹˮ���е�������Ũ������ƽ�������ƶ���Cu2+����ˮ�����������
��7�����0.1mol CuSO4��Һ������ͭ3.2 g��ת�Ƶ���0.1mol���������������ӷŵ磬����0.025mol������ͬʱ��ʹ��Һ�е�����������0.1mol������Һ���Դ���0.05mol Cu2+��SO42-�����ʵ������䣬����0.1mol�����Դ�ʱ��Һ������Ũ�ȵĴ�С��ϵΪc(H+)>c(SO42-)>c(Cu2+)>c(OH-)��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ����˵����ȷ����
����˵����ȷ����