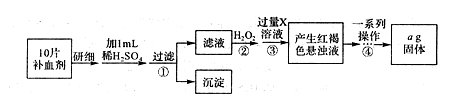
��Ŀ����
.������16�֣�Ϊ��֤�����ԣ�Cl2>Fe3+>SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г������ͼ��м���װ�����ԣ��������Ѽ��飩��
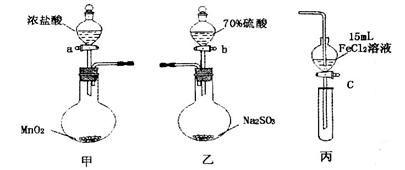
ʵ�鲽�裺
1.�ڼ�װ���У�����a�����ȣ���װ���г�������ɫ����ʱ�����װ�����ӡ�
2.����װ����FeC12��Һ���ʱ��ֹͣ���ȡ�
3.����c��ʹԼ2mL����Һ�����Թ��У�������Һ�е����ӡ�
4.����װ���У�����b���������ž������в���������ͨ��������װ�ñ�ƺ����Һ�У�һ��ʱ���ֹͣ��
5.���±����Թܣ�����c��ʹԼ2mL����Һ�����Թ��У�������Һ�е����ӡ�
�ش��������⣺
��1�����з�����Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
��2����70%��������ȡSO2����Ӧ���ʱ���98%������죬ԭ����__________________��
��3��ʵ���У�֤��������Fe3+��SO2�����ӷ���ʽΪ_____________________________��
��4����I��II��III����ͬѧ�ֱ����������ʵ�飬ʵ�������£�

����ʵ����һ���ܹ�֤��������:Cl2>Fe3+>SO2����___________���á�I������II������III�����Żش𣩡�
��5����Ҫ�����ϼͱ�װ��֤��������Ϊ��Cl2> Fe3+> I2�Ľ��ۣ�����Ϊ��
��������©���м��������Լ�_________��_________��һ���ܼ�__________��
�ڽ���װ���в�����Cl2����ͨ����У��۲����©������Һ��ɫ�仯��
������۲쵽������Һ_______________________________________�������ȷ��
��ֹͣͨ��Cl2��

ʵ�鲽�裺
1.�ڼ�װ���У�����a�����ȣ���װ���г�������ɫ����ʱ�����װ�����ӡ�
2.����װ����FeC12��Һ���ʱ��ֹͣ���ȡ�
3.����c��ʹԼ2mL����Һ�����Թ��У�������Һ�е����ӡ�
4.����װ���У�����b���������ž������в���������ͨ��������װ�ñ�ƺ����Һ�У�һ��ʱ���ֹͣ��
5.���±����Թܣ�����c��ʹԼ2mL����Һ�����Թ��У�������Һ�е����ӡ�
�ش��������⣺
��1�����з�����Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
��2����70%��������ȡSO2����Ӧ���ʱ���98%������죬ԭ����__________________��
��3��ʵ���У�֤��������Fe3+��SO2�����ӷ���ʽΪ_____________________________��
��4����I��II��III����ͬѧ�ֱ����������ʵ�飬ʵ�������£�

����ʵ����һ���ܹ�֤��������:Cl2>Fe3+>SO2����___________���á�I������II������III�����Żش𣩡�
��5����Ҫ�����ϼͱ�װ��֤��������Ϊ��Cl2> Fe3+> I2�Ľ��ۣ�����Ϊ��
��������©���м��������Լ�_________��_________��һ���ܼ�__________��
�ڽ���װ���в�����Cl2����ͨ����У��۲����©������Һ��ɫ�仯��
������۲쵽������Һ_______________________________________�������ȷ��
��ֹͣͨ��Cl2��
33.�𰸣�16�֣�
��1��MnO2 + 4 HCl��Ũ��= MnCl2 + Cl2��+ 2H2O �����ȣ���2�֣�����д�����ȡ�����Ũ�����÷֣�����������ſ�1�֣�
��2��70%��H2SO4��98%��H2SO4����̶ȴ���Һ��H+Ũ�ȴ�Ӧ�ٶȿ� ��2�֣���ֻ��70%��H2SO4��Һ��Һ��H+Ũ�ȴ�Ҳ�ɵ÷֣�
��3��2Fe3+ + SO2 + 2H2O = 2Fe2+ + SO32- + 4H+��2�֣�
��4����͢� ��4�֣���2�֣�
��5����FeI2��KSCN��CCl4��3�֣�
���²㣨CCl4�㣩������ɫ��Ϊ�Ϻ�ɫ��1�֣������ϲ���Һ��dz��ɫ��1�֣���Ϊ��ɫ��1�֣���
��1��MnO2 + 4 HCl��Ũ��= MnCl2 + Cl2��+ 2H2O �����ȣ���2�֣�����д�����ȡ�����Ũ�����÷֣�����������ſ�1�֣�
��2��70%��H2SO4��98%��H2SO4����̶ȴ���Һ��H+Ũ�ȴ�Ӧ�ٶȿ� ��2�֣���ֻ��70%��H2SO4��Һ��Һ��H+Ũ�ȴ�Ҳ�ɵ÷֣�
��3��2Fe3+ + SO2 + 2H2O = 2Fe2+ + SO32- + 4H+��2�֣�
��4����͢� ��4�֣���2�֣�
��5����FeI2��KSCN��CCl4��3�֣�
���²㣨CCl4�㣩������ɫ��Ϊ�Ϻ�ɫ��1�֣������ϲ���Һ��dz��ɫ��1�֣���Ϊ��ɫ��1�֣���
��������� ��1�����ݼ�װ���е�������ʾ�������жϸ�װ����ʵ�����Ʊ������ķ���װ�ã����Ը�װ���з����ķ�Ӧ��MnO2 + 4 HCl��Ũ��= MnCl2 + Cl2��+ 2H2O��
��2��װ������ʵ�����Ʊ�SO2�ķ���װ�ã��÷�Ӧ��ԭ���������ѻӷ�������ǿ�������Ʊ����Խ������ֽ�������ᣬ����Ҫ�Ͽ��Ƶý϶�������ᣨSO2������Ҫ��Һ�к��е������ӵ�Ũ�Ƚϴ����Զ��ڲ�ͬŨ�ȵ�������ԣ������Ũ��С������̶�Խ��������70%��������ȡSO2����Һ��H+Ũ�ȴ�Ӧ���ʱ���98%������졣
��3������֤��������Fe3+��SO2 �����ڱ�Ƶ��Ȼ�������ͨ��SO2��SO2�ֱ�Fe3+ ���������Է�Ӧ�����ӷ���ʽΪ2Fe3+ + SO2 + 2H2O =" 2" Fe3+ + SO32- + 4H+ ��
��4��I��ͬѧ����õ��IJ���3��Һ�Ⱥ���Fe3+�ֺ���Fe2+ ��˵����Һ�е�Fe3+ �������������ã���һ�������й��������������Բ���5�м���SO42- ��Ҳһ������Fe3+ ����SO2���ã���˿��Եó�����������:Cl2>Fe3+>SO2 ��III��ͬѧ���IJ���3��Һֻ��Fe3+����Fe2+����˵����Һ�е�Fe3+ �������������ã�������5�м���Fe2+��Ҳ����˵����Һ��Fe2+ ��SO2��ԭFe3+ ���ã����Կ��Եó�������:Cl2>Fe3+>SO2 �Ľ��ۡ�II��ͬѧ���IJ���3��Һֻ��Fe3+����Fe2+����III��ͬѧ���ƣ���Һ�ж��п����ܽ��й�������������˲���5�м���SO42- ���п������ܽ������еĹ�����������SO2���ã����Բ���˵��������Fe3+>SO2 �����ѡI��III��
��5����Ҫ�����ϼͱ�װ��֤��������Ϊ��Cl2> Fe3+> I2�Ľ��ۣ������Ҫ�ڷ�Һ©���м��뺬��Fe2+ ��I-���Լ������Կ���ѡ��FeI2 ����Һ��֤������������Fe3+�� I2 ����Ҫ��������Ե��Լ�KSCN���л��ܼ���CCl4�����۲�ֲ����Һ���ϲ�dz��ɫ��죬�²����Ϻ�ɫ���������ȷ��

��ϰ��ϵ�д�
�����Ŀ



 R2Cu���л��ࣩ+ 2H����ˮ�ࣩ
R2Cu���л��ࣩ+ 2H����ˮ�ࣩ