��Ŀ����
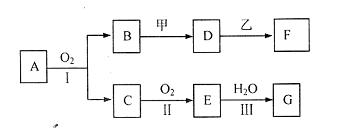
��10�֣�X��Y��Z��W���������������ת����ϵ������X��W���ʣ�Y��ZΪ������,δ�г���Ӧ��������
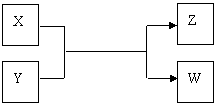
����Z�������г��õĵ�ζƷ��W��������Һ��������
��1�������£�X����ɫ�� ��
��2����ҵ��Z�ж�����;���û�ѧ����ʽ��ʾZ��һ����; ��
��3�����������õ�Z�����˵���أ�����X��Y��Һ��Ӧʱ���Եõ�һ�ֵ����Σ��˷�Ӧ�����ӷ���ʽ�� ��
����X�ǹ�ҵ���������Ľ������ʣ�Z��һ�־��д��Եĺ�ɫ���壬��
��1�� X��Y��Ӧ�Ļ�ѧ����ʽ�� ��
X��Y��Ӧ�Ļ�ѧ����ʽ�� ��
��2����������װ��ֻ����Z + W=" X" + Y��Ӧ���г�װ��δ��������
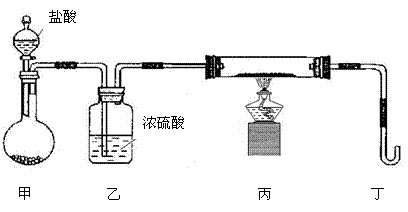
����ɴ�ʵ���жಽ���������������ǣ�
��a����ȼ�ƾ� �� b���μ����� c���������崿��
�� b���μ����� c���������崿��
�������������Ⱥ�˳���� ������ĸ����
������װ����Ҫ�Ľ��������ǣ������������� ��
��3���� 3.48 gZ����50 mL4 mol/L��ϡHNO3�г�ַ�Ӧ������112 mL��NO����״��������Ӧ�����Һ�еμ�NaOH��Һ�ܲ�������������������࣬������Ҫ����2 mol/L��NaOH��Һ mL (��ȷ��0.1)��

����Z�������г��õĵ�ζƷ��W��������Һ��������
��1�������£�X����ɫ�� ��
��2����ҵ��Z�ж�����;���û�ѧ����ʽ��ʾZ��һ����; ��
��3�����������õ�Z�����˵���أ�����X��Y��Һ��Ӧʱ���Եõ�һ�ֵ����Σ��˷�Ӧ�����ӷ���ʽ�� ��
����X�ǹ�ҵ���������Ľ������ʣ�Z��һ�־��д��Եĺ�ɫ���壬��
��1��
 X��Y��Ӧ�Ļ�ѧ����ʽ�� ��
X��Y��Ӧ�Ļ�ѧ����ʽ�� ����2����������װ��ֻ����Z + W=" X" + Y��Ӧ���г�װ��δ��������

����ɴ�ʵ���жಽ���������������ǣ�
��a����ȼ�ƾ�
 �� b���μ����� c���������崿��
�� b���μ����� c���������崿���������������Ⱥ�˳���� ������ĸ����
������װ����Ҫ�Ľ��������ǣ������������� ��
��3���� 3.48 gZ����50 mL4 mol/L��ϡHNO3�г�ַ�Ӧ������112 mL��NO����״��������Ӧ�����Һ�еμ�NaOH��Һ�ܲ�������������������࣬������Ҫ����2 mol/L��NaOH��Һ mL (��ȷ��0.1)��
(10��)
��
��1������ɫ (1��)
��2��2NaCl +2 H2O H2��+ Cl2�� + 2 NaOH (2��)
H2��+ Cl2�� + 2 NaOH (2��)
��3��3Cl2 + I��+ 3 H2O ="=" 6 Cl- + IO3��+ 6 H+ (2��)
(2��)
��
��1��3 Fe + 4 H2O��g�� Fe3O4 + 4 H2 (2��)
Fe3O4 + 4 H2 (2��)
��2����b c a (1��)
���ڼס���װ���м�����һ��װ��ˮ��ϴ ��ƿ (1��)
��ƿ (1��)
��3��97.5 (1��) (10��)
��
��1������ɫ (1��)
��2��2NaCl +2 H2O H2��+ Cl2�� + 2 NaOH (2��)
H2��+ Cl2�� + 2 NaOH (2��)
��3��3Cl2 + I��+ 3 H2O ="=" 6 Cl- + IO3��+ 6 H+ (2��)
(2��)
��
��1��3 Fe + 4 H2O��g�� Fe3O4 + 4 H2 (2��)
Fe3O4 + 4 H2 (2��)
��2����b c a (1��)
���ڼס���װ���м�����һ��װ��ˮ��ϴ ��ƿ (1��)
��ƿ (1��)
��3��97.5 (1��)
��
��1������ɫ (1��)
��2��2NaCl +2 H2O
 H2��+ Cl2�� + 2 NaOH (2��)
H2��+ Cl2�� + 2 NaOH (2��)��3��3Cl2 + I��+ 3 H2O ="=" 6 Cl- + IO3��+ 6 H+
 (2��)
(2��)��
��1��3 Fe + 4 H2O��g��
 Fe3O4 + 4 H2 (2��)
Fe3O4 + 4 H2 (2��)��2����b c a (1��)
���ڼס���װ���м�����һ��װ��ˮ��ϴ
 ��ƿ (1��)
��ƿ (1��)��3��97.5 (1��) (10��)
��
��1������ɫ (1��)
��2��2NaCl +2 H2O
 H2��+ Cl2�� + 2 NaOH (2��)
H2��+ Cl2�� + 2 NaOH (2��)��3��3Cl2 + I��+ 3 H2O ="=" 6 Cl- + IO3��+ 6 H+
 (2��)
(2��)��
��1��3 Fe + 4 H2O��g��
 Fe3O4 + 4 H2 (2��)
Fe3O4 + 4 H2 (2��)��2����b c a (1��)
���ڼס���װ���м�����һ��װ��ˮ��ϴ
 ��ƿ (1��)
��ƿ (1��)��3��97.5 (1��)
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ZX2��Ӧ����һ�ֻ���
ZX2��Ӧ����һ�ֻ��� ��Y2ZX3����֪����Ԫ��ԭ�ӵĵ�������Ϊ25����Z��Y��ԭ������֮�ͱ�X��ԭ��������2������1��Zԭ�ӵ������������Ǵ�����������2�����Իش�
��Y2ZX3����֪����Ԫ��ԭ�ӵĵ�������Ϊ25����Z��Y��ԭ������֮�ͱ�X��ԭ��������2������1��Zԭ�ӵ������������Ǵ�����������2�����Իش�