��Ŀ����
����Ŀ����⾫��ͭ������������Ҫ��Ag��Au�ȹ��ؽ����������ǴӾ���ͭ���������л��������������ͼ��
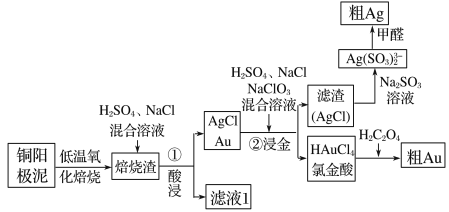
(1)�Ƚ���(HAuCl4)�е�Au�Ļ��ϼ�Ϊ________��
(2)ͭ����������ʱ�����á����±��ա��������á����±��ա���ԭ����_____________________��
(3)�����������ڡ��������ʱ������Ӧ�����ӷ���ʽΪ______________________________________��
(4)���ڽ��𡱷�Ӧ�У�H2SO4������Ϊ___________________________________________���ò���ķ�������У���Ҫ�����õ�AgCl����ˮϴ����������ж�AgCl�Ѿ�ϴ�Ӹɾ��� _____________________________________________________��
(5)�Ƚ���(HAuCl4)��pHΪ2��3�������±����ỹԭΪAu��ͬʱ�ų�������̼���壬��÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________��
(6)��ȩ��ԭ����������ͨ�����ڽ����������¼������������½��У���ȩ������Ϊ̼��������ӣ���÷�Ӧ�����ӷ���ʽΪ___________________________________________��
���𰸡���3 ���±���ʱ�����ɵ�Ag2O�ַֽ�ΪAg��O2(��2Ag2O![]() 4Ag��O2��) Ag2O��2H����2Cl��=2AgCl��H2O �ṩH������ǿNaClO3�������� ȡ���һ��ϴ��Һ�������Թ��У�����Ba(NO3)2��Һ����û�а�ɫ�������������Ѿ�ϴ�Ӹɾ�����֮������Ҫ����ϴ�� 2HAuCl4��3H2C2O4=2Au����8HCl��6CO2�� 4Ag(SO3)23-��HCHO��5OH��=4Ag����8SO32-��3H2O��HCO3-
4Ag��O2��) Ag2O��2H����2Cl��=2AgCl��H2O �ṩH������ǿNaClO3�������� ȡ���һ��ϴ��Һ�������Թ��У�����Ba(NO3)2��Һ����û�а�ɫ�������������Ѿ�ϴ�Ӹɾ�����֮������Ҫ����ϴ�� 2HAuCl4��3H2C2O4=2Au����8HCl��6CO2�� 4Ag(SO3)23-��HCHO��5OH��=4Ag����8SO32-��3H2O��HCO3-
��������
ͭ����������������գ�Ȼ�����������Ȼ��ƵĻ������ˣ�����ΪAgCl��Au���������м����ᡢNaCl�������ƵĻ�����������ΪAgCl����ҺΪ�Ƚ��ᣬAgCl�м�Na2SO3��Һת��ΪAg��SO3��23-������HCHO��ԭ�õ���Ag��HAuCl4��Һ�м�H2C2O4���ɴֽ�
��1���Ƚ��ᣨHAuCl4����ClΪ-1�ۣ�HΪ+1�ۣ���Au�Ļ��ϼ�Ϊ+3���ʴ�Ϊ��+3��
��2�����±���ʱ��Ag������ת��ΪAg2O������ʱ���������ֽ�������Ag���������ʴ�Ϊ�����±���ʱ�����ɵ�Ag2O�ַֽ�ΪAg��O2(��2Ag2O![]() 4Ag��O2��)��
4Ag��O2��)��
��3�����ʱ�������������ᷴӦ�����Ȼ�������Ӧ�����ӷ���ʽΪ��Ag2O+2H++2Cl-=2AgCl+H2O���ʴ�Ϊ��Ag2O+2H++2Cl-=2AgCl+H2O��
��4�����ڽ�������Ӧ�У����������£�Au�������Ʒ�Ӧ�����������ṩH+����ǿNaClO3�������ԣ���Һ�к�����������ӣ��������һ��ϴҺ���Ƿ�����������ӣ������Ϊȡ���һ��ϴ��Һ�������Թ��У�����Ba��NO3��2��Һ����û�а�ɫ�������������Ѿ�ϴ�Ӹɾ�����֮������Ҫ����ϴ�ӣ��ʴ�Ϊ���ṩH������ǿNaClO3�������� ��ȡ���һ��ϴ��Һ�������Թ��У�����Ba(NO3)2��Һ����û�а�ɫ�������������Ѿ�ϴ�Ӹɾ�����֮������Ҫ����ϴ�ӣ�
��5���Ƚ�������ᷴӦ����Au��HCl�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��2HAuCl4+3H2C2O4=2Au+8HCl+6CO2�����ʴ�Ϊ��2HAuCl4+3H2C2O4=2Au+8HCl+6CO2����
��6�������������£�Ag��SO3��23-����ȩ��ԭΪAg��ͬʱ����̼��������ӣ���Ӧ�����ӷ���ʽΪ��4Ag��SO3��23-+HCHO+5OH-=4Ag+8SO32-+3H2O+HCO3-��

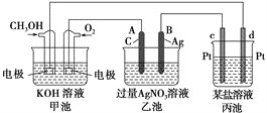
����Ŀ��ijͬѧ̽��Cu��NO�ķ�Ӧ���������ϣ���Cu��NO��Ӧ������CuO��N2�������������£�NO��NO2�C������MnO4�C��Ӧ����NO3�C��Mn2+
��1��ʵ��������Cu��ϡHNO3�Ʊ�NO��д����Ӧ�Ļ�ѧ����ʽ_____________��
��2��ѡ����ͼ��ʾװ�����Cu��NO��ʵ�顣(�г�װ����) ʵ�鿪ʼǰ����װ����ͨ��һ��ʱ���N2���ش��������⣺

��ʹ��ͭ˿���ŵ���_____________________װ��E������Ϊ_______________��
��װ��C��ʢ�ŵ�ҩƷ������_________��
��װ��D�е�������_______________��װ��F�з�Ӧ�����ӷ���ʽ��_______________��
��3���ⶨNaNO2��NaNO3 �����Һ��NaNO2��Ũ�ȡ� ȡ25.00mL�����Һ����ƿ�У���0.1000mol��L��1����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
����� | 1 | 2 | 3 | 4 |
����KMnO4��Һ���/mL | 20.90 | 20.12 | 20.00 | 19.88 |
����һ��ʵ�����ݳ����쳣����������쳣��ԭ�������_________������ĸ���ţ���
a����ƿϴ����δ����
b����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
c���ζ��յ�ʱ���Ӷ���
d������KMnO4��Һ�к��������������Լ�
e����ƿϴ�����ô���Һ��ϴ
������KMnO4��Һ�ζ�����������Һ�����ӷ���ʽΪ___________________��
��NaNO2 �����ʵ���Ũ��Ϊ__________