��Ŀ����
���й��ڻ�ѧʵ��ġ�Ŀ��-����-����-���ۡ�����������ȷ���ǣ� ��
| | ʵ��Ŀ�� | �����Լ����������ͼʾ�� | ʵ������ | ʵ����� |
| A | ����ϡ���������������Һ�Ƿ�ǡ����ȫ��Ӧ | �ڷ�Ӧ�����Һ�еμ���ɫ��̪��Һ | ���������� | ǡ����ȫ��Ӧ |
| B | �жϺ�ˮ������ˮ | �����ᾧ | Һ����ʧ�������� | ��Һ��Ϊ����ˮ |
| C | ������Һ���Ƿ�̼������ӻ�̼��������� | ����ϡ�����ٽ�����ͨ��ʯ��ˮ�� | ����ɫ��ζ�����������ʯ��ˮ���а�ɫ�����γ� | ��Һ��һ����̼������ӻ�̼��������� |
| D | �ⶨ������������������� | 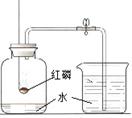 | ��ȫȼ�պ���ȴ�����£����ɼУ�����ˮ�����ԼΪ����ƿ�п��������1/5 | ����Լռ���������1/5 |
A
�������������ϡ���������������Һ�Ƿ�ǡ����ȫ��Ӧ���ڷ�Ӧ�����Һ�еμ���ɫ��̪��Һ�����������������кͣ�Ҳ�������������Ϊ���ʹ��̪��ɫ��ʵ��ġ�Ŀ��-����-����-���ۡ�����������ȷ����A�����жϺ�ˮ������ˮ�����������ᾧ��Һ����ʧ�������˵��Ϊ��Һ��Ϊ����ˮ��ʵ��ġ�Ŀ��-����-����-���ۡ���������ȷ����B��ȷ��������Һ���Ƿ�̼������ӻ�̼��������ӣ�����ϡ�����ٽ�����ͨ��ʯ��ˮ�У���������������ɰ�ɫ����������Һ��һ����̼������ӻ�̼��������ӣ�ʵ��ġ�Ŀ��-����-����-���ۡ���������ȷ����C��ȷ�����ⶨ�����������������������ͼ��ȫȼ�պ���ȴ�����£����ɼУ�����ˮ�����ԼΪ����ƿ����������֮һ��������Լռ������������֮һ��ʵ��ġ�Ŀ��-����-����-���ۡ���������ȷ����D��ȷ��

��ϰ��ϵ�д�
�����Ŀ
 BCl3����3H2���������������������ƣ�Ҳ��������������Һ��Ӧ��
BCl3����3H2���������������������ƣ�Ҳ��������������Һ��Ӧ��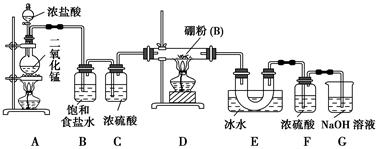
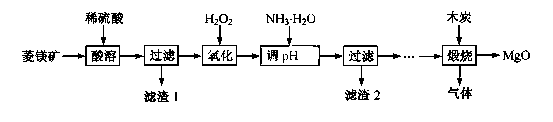
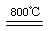 2MgO+2SO2��+CO2��
2MgO+2SO2��+CO2��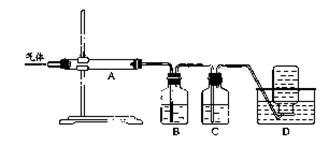
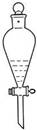
 N2O4��g�� ��H<0
N2O4��g�� ��H<0 (CH3CH2CH2CH2)2O+H2O
(CH3CH2CH2CH2)2O+H2O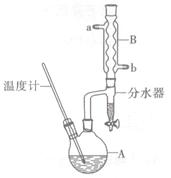
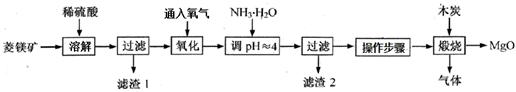
 2MgO+2SO2��+CO2���� MgSO4+C
2MgO+2SO2��+CO2���� MgSO4+C MgO+S��+3CO����
MgO+S��+3CO����