��Ŀ����
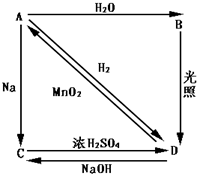
��ͼ��ʾ����������1��20��Ԫ���в���Ԫ����ɵĵ��ʻ��仯���ͼ�в��ַ�Ӧ�����Ͳ���δ�г�����֪C��D��E��H�dz�����������DΪ���ʡ���Ӧ�ں͢��ǻ��������е���Ҫ��Ӧ����Ӧ����ʵ�����Ʊ�����C����Ҫ��������Ӧ����J��ʧЧ��Ӧԭ����
��ش��������⣺
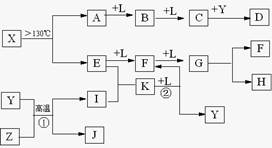
������L�ĵ���ʽΪ____________��E�ĽṹʽΪ_______________��
��D����Ԫ��λ�����ڱ�__________________������D�Ĺ�ҵ����Ϊ_______________��
�Ƿ�Ӧ�ݵĻ�ѧ����ʽΪ___________________________________________��
��Ӧ�����ӷ���ʽΪ___________________________________________��
������A������Ԫ����ɣ�1molA��ˮ��Ӧ������1molB��2molC��A�Ļ�ѧʽΪ________��

��ش��������⣺
������L�ĵ���ʽΪ____________��E�ĽṹʽΪ_______________��
��D����Ԫ��λ�����ڱ�__________________������D�Ĺ�ҵ����Ϊ_______________��
�Ƿ�Ӧ�ݵĻ�ѧ����ʽΪ___________________________________________��
��Ӧ�����ӷ���ʽΪ___________________________________________��
������A������Ԫ����ɣ�1molA��ˮ��Ӧ������1molB��2molC��A�Ļ�ѧʽΪ________��
��  O=C=O��2�֣��Ƶ�������VIIA�壬�ȼҵ��2�֣�
O=C=O��2�֣��Ƶ�������VIIA�壬�ȼҵ��2�֣�
��Ca(OH)2��2NH4Cl CaCl2��2NH3����2H2O��2�֣�
CaCl2��2NH3����2H2O��2�֣�
Ca2++2ClO��+CO2+H2O=CaCO3��+2HClO��2�֣� ��CaCN2��2�֣�
 O=C=O��2�֣��Ƶ�������VIIA�壬�ȼҵ��2�֣�
O=C=O��2�֣��Ƶ�������VIIA�壬�ȼҵ��2�֣���Ca(OH)2��2NH4Cl
 CaCl2��2NH3����2H2O��2�֣�
CaCl2��2NH3����2H2O��2�֣�Ca2++2ClO��+CO2+H2O=CaCO3��+2HClO��2�֣� ��CaCN2��2�֣�
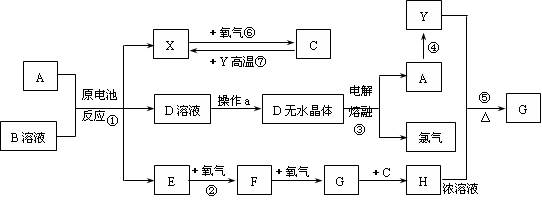
����ͻ�ƿ�Ϊ����Ӧ����J��ʧЧ��Ӧԭ������ѧ��ֻ��Ư�۷������⣬��JΪCa(ClO)2����֪DΪ�������嵥�ʣ�D+I��J+K+H2O,�ҷ�Ӧ�ڢ��ǹ�ҵ�����е���Ҫ��Ӧ������֪��DΪCl2��IΪCa(OH)2,���ǹ�ҵ��Ư�۵���Ҫ��Ӧ����F+H2O��I����FΪCaO�� BΪCaCO3����Ϊ��ҵ����ʯ��ʯ�����ɷ�Ӧ����ʵ�����Ʊ�����C����Ҫ������I+G ��C+K+H2O��CΪ������GΪNH4Cl,C+H��NH4Cl������HΪHCl�������ѵ���ȷ������A��ɣ���1molA��ˮ��Ӧ����1molB��2molC֪��A+H2O��CaCO3+2NH3,��ԭ���غ�֪H2Oϵ��Ϊ3����AΪCaCN2��

��ϰ��ϵ�д�
�����Ŀ
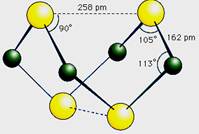
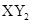 �ͻ������X��Y ��ԭ������֮�����Ϊ2��5
�ͻ������X��Y ��ԭ������֮�����Ϊ2��5