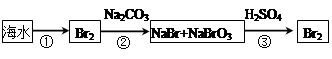��Ŀ����
��ҵ���������������ͼ���£���ش��������⣺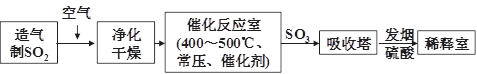
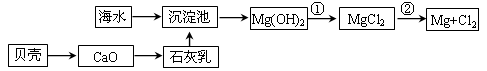
��1���������������Ի�����Ϊԭ�ϣ������ڹ����������������Ϊԭ�ϣ������� ��
��2��������������Ӧ��ǰ�辻����ԭ���� ��
��3���ڴ���Ӧ����ͨ��ʹ�ó�ѹ���ڴ�������SO2��ת����Ϊ90%�����Dz��ַ�����Ҳ�ȡ��ѹ��������ȡSO3����ȡ��ѹ��ʩ��Ŀ�ij��˼ӿ췴Ӧ�����⣬������ ���Ӷ��������Ч�ʡ�
��4����ҵ�����г��ð����ᷨ����β�������Դﵽ������Ⱦ���������õ�Ŀ�ġ��û�ѧ����ʽ��ʾ�䷴Ӧԭ���� ��
��5�������Ṥҵ�⣬�������ҵ������������صĹ�ҵ������������ȷ���� ��
A����ˮ���壺��ˮŨ��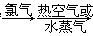 ������ ������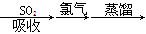 Һ�� Һ�� |
B����ˮ��þ����̲����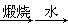 ʯ��ˮ ʯ��ˮ MgO MgO þ þ |
C����ҵ��������� NO2 NO2 �����β������ �����β������ |
D����ҵ�ϳɰ�����Ȼ�� ���� ���� NH3��H2��N2 NH3��H2��N2 �� �� |
��15�֣���1���Ի�����Ϊԭ�ϵ������в����ķ�����̫�࣬�����ɱ��ߣ�2�֣�
��2����ֹ�����ж���2�֣� ��3��ʹƽ�����������ƶ������������SO2��ת���ʣ�2�֣�
��4��SO2+NH3+H2O��NH4HSO3��2�֣� 2NH4HSO3+H2SO4��(NH4)2SO4+2H2O+2SO2����3�֣�
��SO2+2NH3+H2O��(NH4)2SO3��2�֣� (NH4)2SO3+H2SO4��(NH4)2SO4+H2O+SO2����3�֣�
��5��A D����2�֣�
���������������1��������������̫�࣬����Ի�����Ϊԭ�ϵ������в����ķ�����̫�࣬�����ɱ��ߡ�����ȼ�յIJ���ֻ��SO2�����ڴ�����������ڹ����������������Ϊԭ�ϡ�
��2���������ɵ������к��кܶ����ʣ�������ɴ����ж�������������������Ӧ��ǰ�辻����ԭ���Ƿ�ֹ�����ж���
��3�����ݷ���ʽ2SO2��O2 2SO3��֪���÷�Ӧ�������С�Ŀ��淴Ӧ���������ѹǿ����ʹƽ�����������ƶ������������SO2��ת���ʡ�
2SO3��֪���÷�Ӧ�������С�Ŀ��淴Ӧ���������ѹǿ����ʹƽ�����������ƶ������������SO2��ת���ʡ�
��4�������Ǽ������壬SO2���������壬��˰�����������SO2������������炙�������李����ɵ���������炙������������ϡ���ᷴӦ��ת��ΪSO2���Ӷ��ﵽĿ�ġ��йط�Ӧ�ķ���ʽΪSO2+NH3+H2O��NH4HSO3��2NH4HSO3+H2SO4��(NH4)2SO4+2H2O+2SO2����SO2+2NH3+H2O��(NH4)2SO3��(NH4)2SO3+H2SO4��(NH4)2SO4+H2O+SO2����
��5��A���������������ԣ������������������ɵ����壬Ȼ��������SO2�����������Դﵽ������Ŀ�ġ���������������������Խ������ӻ�ԭ���ɵ����壬���ѡ��A����ʵ�ֺ�ˮ���壬A��ȷ��B����ҵ����ͨ��������ڵ��Ȼ�þ��ұ������þ�������������۵�̫�ߣ���Ҫ���Ĵ�����Դ��B����ȷ��C�������еĵ����ڷŵ������������������ֻ������NO���ò���NO2��C����ȷ��D�������ȵ���Ҫ�ɷ��Ǽ��飬��һ�������¿����������������뵪�����Ժϳɰ��������ѡ��D����ʵ�ֹ�ҵ�ϳɰ���D��ȷ����ѡAD��
���㣺���鹤ҵ�Ʊ�������й��ж�

���н���ұ���ķ�Ӧԭ������ȷ����
A��2AlCl3(����)  2Al+3Cl2�� 2Al+3Cl2�� |
B��MgO+H2 Mg+H2O Mg+H2O |
C��ZnO+CO  Zn+CO2 Zn+CO2 |
D��2CuO  2Cu+O2�� 2Cu+O2�� |
�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�):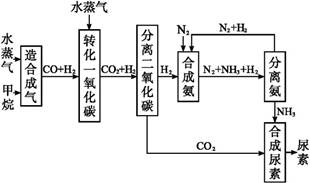
����д���пհ�:
(1)��֪0.5 mol�����0.5 molˮ������t�桢p kPaʱ,��ȫ��Ӧ����һ����̼������(�ϳ���),������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ����������������
(2)����������,��ҵ�Ϸ���H2��CO2�����ķ�������������
| A���������ͨ������������Һ,������Һ�м������� |
| B���������ѹ��ȴ,ʹCO2Һ�� |
| C��������ð�ˮϴ�� |
| D���������ͨ��ʯ�ҽ���,Ȼ��������չ��� |
(4)������������Դ����������߾���Ч��,Ҳ�Ƕ���ᡢ��ȫ���ฺ��ı���,�����߶κͼ�ͷ����ͼ�е���������������Դ�����
�ҹ����������ձ��ȹ��Ҷ������Ƴ�һ���մɲ��ͻ������ֲ��ͻ��ķ���������������������һ�������Ҳ��״��ȵIJ���������ģ����ֲ�����(����)��
| A���������մ� | B���������մ� |
| C�����ά | D�������� |