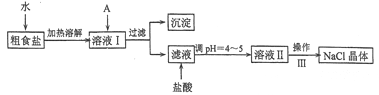
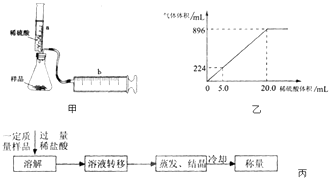
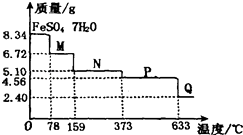
��Ŀ����
����Ŀ����ѧ�ܰ������Ǹ��õ���ʶ���������� �ټ�������δϴ�����ɣ�����һ��ʱ�����ֺ��ɫ��ߣ�����Ҫ��ԭ��������������ʴ��
�ڳ˳���ֹЯ����Ʒ�IJ��ֱ�ʶ��ͼ��ʾ����Ũ���ᡢʳ�Ρ����͡�ʯ��������Ʒ�У�����Я���ϳ����� ��
�۹������ᣨ ![]() ���ֽ�Ϊ�������������ϡ�ܳ����ڲ;���е����������������ֽ�Ļ�ѧ����ʽ �� ijθҩ����Ҫ�ɷ���AlbMgc��OH��mCO3 �� ����������θ����࣬д��������θ�ᷴӦ�Ļ�ѧ����ʽ ��
���ֽ�Ϊ�������������ϡ�ܳ����ڲ;���е����������������ֽ�Ļ�ѧ����ʽ �� ijθҩ����Ҫ�ɷ���AlbMgc��OH��mCO3 �� ����������θ����࣬д��������θ�ᷴӦ�Ļ�ѧ����ʽ �� 
���𰸡��绯ѧ��Ũ��������ͣ�2C2H4O3 ![]() 2C2H4O2+O2����2AlbMgc��OH��mCO3+��6b+4c��HCl=2bAlCl3+2cMgCl2+��3b+2c+m��H2O+2CO2��
2C2H4O2+O2����2AlbMgc��OH��mCO3+��6b+4c��HCl=2bAlCl3+2cMgCl2+��3b+2c+m��H2O+2CO2��
���������⣺�������к���Fe��C��Fe��C�͵������Һ����ԭ��أ�Fe��ʧ���������������ٱ���ʴ��������������ʴ���ڵ绯ѧ��ʴ�����Դ��ǣ��绯ѧ��ʴ���ڳ���������ͨ���߽�ֹЯ����ȼ�ױ�����ʴ�Ժ��ж���Ʒ����Ũ�������ڸ�ʴ��ҩƷ������������ȼ�ױ���Һ�壬�ʾ�����Я���ϳ������Դ��ǣ�Ũ��������ͣ��۹��������¶��Ը��ֽ�ų����������ɴ��ᣨC2H4O2������Ӧ�Ļ�ѧ����ʽΪ��2C2H4O3 ![]() 2C2H4O2+O2����AlbMgc��OH��mCO3�Ǽ�ʽ̼���Σ����ܺ����ᷴӦ����AlCl3��MgCl2��H2O��CO2 �� ��ѧ����ʽΪ2AlbMgc��OH��mCO3+��6b+4c�� HCl=2b AlCl3+2cMgCl2+��3b+2c+m��H2O+2CO2���� ���Դ��ǣ�2CH3COOH��2CH3COOH+O2����2AlbMgc��OH��mCO3+��6b+4c�� HCl=2b AlCl3+2cMgCl2+��3b+2c+m��H2O+2CO2����
2C2H4O2+O2����AlbMgc��OH��mCO3�Ǽ�ʽ̼���Σ����ܺ����ᷴӦ����AlCl3��MgCl2��H2O��CO2 �� ��ѧ����ʽΪ2AlbMgc��OH��mCO3+��6b+4c�� HCl=2b AlCl3+2cMgCl2+��3b+2c+m��H2O+2CO2���� ���Դ��ǣ�2CH3COOH��2CH3COOH+O2����2AlbMgc��OH��mCO3+��6b+4c�� HCl=2b AlCl3+2cMgCl2+��3b+2c+m��H2O+2CO2����
�����㾫����ͨ��������ý����ĵ绯ѧ��ʴ����������ս����ı�����Ϳ�����㣻���ָ���ı�������ڲ��ṹ��ʹ���ȶ���������������������������һ�ָ�Ϊ���õĽ�����Ҫ�����Ľ�������ԭ��أ���ӵ�Դ�������Խ����⣮