��Ŀ����
��15�֣��Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������
��1������Ϊ���ı��洦����һ�ַ�����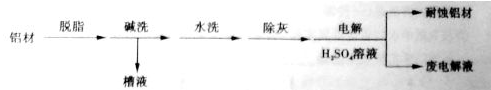
��ϴ��Ŀ���dz�ȥ���ı������Ȼ����Ĥ����ϴʱ��������ð����ԭ����______�������ӷ���ʽ��ʾ����Ϊ����ϴ��Һ�е���Ԫ���Գ�����ʽ���գ�������Һ�м��������Լ��е�______ ��
a��NH3 b��CO2 c��NaOH d��HNO3
��2����ͭ�ɷ�ֹ����Ʒ��ʴ�����ʱ��ͭ������ʯī��������ԭ���� ______ ��
��3��������ͼװ�ã�����ģ�����ĵ绯ѧ������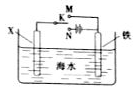
��XΪ̼����Ϊ�������ĸ�ʴ������KӦ����______����
��XΪп������K����M�����õ绯ѧ��������Ϊ_______ ��

����������Ĥȥ������Ҳ��Ӧ��2Al��2OH�D��2H2O=2AlO2�D��3H2����AlO2�D��2H2O��CO2=HCO3�D��Al (OH)3��,ѡC����������ͭ�ɱ���������Cu2�� ����KӦ����N��������ӵ���������������������XΪп�������ã��γ�ԭ��أ�����������������������������

��ϰ��ϵ�д�
�����Ŀ





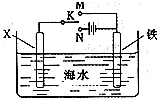 ���������ִ��������ϵ����ǣ�
���������ִ��������ϵ����ǣ�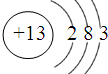
 ��������Ӧ�ù㷺�Ľ���֮һ���ڹ�ũҵ���������Ź㷺��Ӧ�ã��Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������
��������Ӧ�ù㷺�Ľ���֮һ���ڹ�ũҵ���������Ź㷺��Ӧ�ã��Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������