��Ŀ����
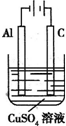 ��������Ӧ�ù㷺�Ľ���֮һ���ڹ�ũҵ���������Ź㷺��Ӧ�ã��Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������
��������Ӧ�ù㷺�Ľ���֮һ���ڹ�ũҵ���������Ź㷺��Ӧ�ã��Խ�����Ʒ���п���ʴ���������ӳ���ʹ����������1���ڽ��е��ǰ��Ҫ�����缫���б��洦�����ǽ���Ƭ�����ȵ�16%NaOH��Һ�а�������ң�Ŀ����
��2������ƬΪ��������H2SO4��Һ�е�⣬ȡ�����ϵ��Һ������NaHCO3�ܽ��������ݺͰ�ɫ����������������ԭ����
��3���ڽ��������γ�һ�����п���ʴ����ĥ�������Ľ�����Ĥ����������Ƭ�ϵ��ͭʱ�����ԭ���Dz���Cu��NO3��2��ҺΪ����ʣ���������������ʵ�ʵĵ�ƹ����У�Ҫ����ƽ����ڶƼ��ϵ��������ʣ�ʹ�����ĶƲ��Ⱦ��ȡ��⻬���ܡ���ײ�����ĸ�����ǿ�����Ե��ͭʱ��ʵ��ʹ�õĵ��Һ��
a��AlCl3
b��CuCl2
c��K6[Cu��P2O7��2]
d��CuSO4
��4������ͼ��ʾװ���У���Һ�����Ϊ100mL����ʼʱ�������Һ��Ũ��Ϊ0.1mol/L������һ��ʱ�������ͨ��0.001mol���ӣ����������ε�ˮ�����Һ����ı仯���������Һ��pHΪ
��������1�������缫���б��洦����ȥ���ı������Ȼ����Ĥ����ϴ��Ŀ���dz�ȥ���ı������Ȼ����Ĥ��������ð����2Al+2OH-+2H2O�T2AlO2-+3H2����
��2����Ϊ�������ᷢ��������Ӧ�������γ�����Ĥ��������ˮ�μӣ����Ե缫��ӦʽΪ��2Al+3H2O-6e-�TAl2O3+6H+��HCO3-��H+��ӦʹH+Ũ�ȼ�С������Al��OH��3������
��3�����ʱ�öƲ����Ϊ�������Ƽ�Ϊ���������жƲ�������ӵ���ҺΪ�������Һ����˲���ͭΪ������ͭ�������ӵ������ͭ����Ũ��С��
��4���������еĵ缫��Ӧ�͵����غ㡢�����Ӻ������������غ����õ���
��2����Ϊ�������ᷢ��������Ӧ�������γ�����Ĥ��������ˮ�μӣ����Ե缫��ӦʽΪ��2Al+3H2O-6e-�TAl2O3+6H+��HCO3-��H+��ӦʹH+Ũ�ȼ�С������Al��OH��3������
��3�����ʱ�öƲ����Ϊ�������Ƽ�Ϊ���������жƲ�������ӵ���ҺΪ�������Һ����˲���ͭΪ������ͭ�������ӵ������ͭ����Ũ��С��
��4���������еĵ缫��Ӧ�͵����غ㡢�����Ӻ������������غ����õ���
����⣺��1�������缫���б��洦����ȥ���ı������Ȼ����Ĥ����Ӧ�����ӷ���ʽΪ��Al2O3+2OH-=H2O+2AlO2-��������ǿ�Ӧ����������2Al+2OH-+2H2O�T2AlO2-+3H2����
�ʴ�Ϊ����ȥ���ı������Ȼ����Ĥ��2Al2O3+2OH-=H2O+2AlO2-��Al+2OH-+2H2O�T2AlO2-+3H2����
��2����Ϊ�������ᷢ��������Ӧ�������γ�����Ĥ��������ˮ�μӣ����Ե缫��ӦʽΪ��2Al+3H2O-6e-�TAl2O3+6H+������NaHCO3��Һ��������ݺͰ�ɫ�����������ڷϵ��Һ�к���Al3+����HCO3-�����˻���ˮ�⣮��HCO3-��H+��ӦʹH+Ũ�ȼ�С������Al��OH��3������
�ʴ�Ϊ��2Al+3H2O-6e-�TAl2O3+6H+��HCO3-��H+��ӦʹH+Ũ�ȼ�С������Al��OH��3������
��3�����ʱ�öƲ����Ϊ�������Ƽ�Ϊ���������жƲ�������ӵ���ҺΪ�������Һ����˲���ͭΪ������ͭ�������ӵ������ͭ����Ũ��С������������ײ�����ĸ�����ǿ���ʴ�Ϊ��C��
��4���������������缫��ӦΪ4OH--4e-=2H2O+O2��������ת��0.001mol�����������Ӽ���0.001mol����Һ������������0.001mol����Һ�������ӵ�Ũ��=
=0.01mol/L��pH=-lg0.01=2���ʴ�Ϊ��2��
�ʴ�Ϊ����ȥ���ı������Ȼ����Ĥ��2Al2O3+2OH-=H2O+2AlO2-��Al+2OH-+2H2O�T2AlO2-+3H2����
��2����Ϊ�������ᷢ��������Ӧ�������γ�����Ĥ��������ˮ�μӣ����Ե缫��ӦʽΪ��2Al+3H2O-6e-�TAl2O3+6H+������NaHCO3��Һ��������ݺͰ�ɫ�����������ڷϵ��Һ�к���Al3+����HCO3-�����˻���ˮ�⣮��HCO3-��H+��ӦʹH+Ũ�ȼ�С������Al��OH��3������
�ʴ�Ϊ��2Al+3H2O-6e-�TAl2O3+6H+��HCO3-��H+��ӦʹH+Ũ�ȼ�С������Al��OH��3������
��3�����ʱ�öƲ����Ϊ�������Ƽ�Ϊ���������жƲ�������ӵ���ҺΪ�������Һ����˲���ͭΪ������ͭ�������ӵ������ͭ����Ũ��С������������ײ�����ĸ�����ǿ���ʴ�Ϊ��C��
��4���������������缫��ӦΪ4OH--4e-=2H2O+O2��������ת��0.001mol�����������Ӽ���0.001mol����Һ������������0.001mol����Һ�������ӵ�Ũ��=
| 0.001mol |
| 0.1L |
���������⿼����������ʡ������ˮ�⡢��⡢����Լ������ĸ�ʴ������֪ʶ�������Ϊ�ۺϣ�����ʱע����պ�Al��AlO2-��Al��OH��3�����ʵ����ʣ����յ�⡢��Ƶ�֪ʶ��ԭ�����˽�����ķ�����ʩ���ѶȲ���Ҫע��֪ʶ�Ļ��ۣ������������

��ϰ��ϵ�д�
�����Ŀ
