��Ŀ����
��ʽ̼��ͭ[Cu2��OH��2CO3]�ǿ�ȸʯ����Ҫ�ɷ֣�����ʱ�ɷֽ�Ϊ����ͭ��������̼��ˮ���������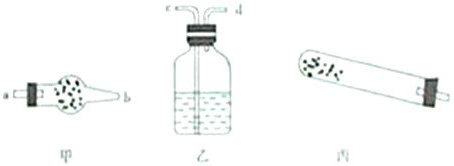
��1��������ͼ��ʾ�����Էֽ�����еķǹ��������������֤�����ʽ̼��ͭӦ�÷���______�У���ס��ҡ��������һ���ʹ�õIJ���������______��
��2������ʽ̼��ͭ�������ľ̿��ϼ��ȣ����ܵõ�ͭ��һ����̼��ˮ�������ʣ��÷�Ӧ�Ļ�ѧ����ʽ��______��
��3��ͭ����Ͻ���;�㷺��������ͭ�ĺϽ��л�ͭ��ͭп�Ͻ𣩡���ͭ��ͭ���Ͻ𣩣�����w g��ͭ��Ʒ�������������������У�����V mL���壨��״���£�������Ʒ��ͭ��п�����ʵ���֮����______���ú�m��V�Ĵ���ʽ��ʾ����
�⣺��1����ʽ̼��ͭӦ�÷��ڱ�װ���У���ȱ�ټ��ȵ������ƾ��ƣ�
�ʴ�Ϊ�������ƾ��ƣ�
��2���Ѽ�ʽ̼��ͭ��C��CuԪ�ػ��ϼ۱仯�������壬���ϼ��ܽ��ͣ�2����2-0��+��4-2��=6�ۣ�̼���ʻ��ϼ�����2�ۣ���̼�ļ�������3����ʽ̼��ͭ�Ļ�ѧ��������1
��ƽ��Ӧ����ʽ��3C+Cu2��OH��2CO3 2Cu+4CO��+H20����
2Cu+4CO��+H20����
�ʴ�Ϊ��4C+Cu2��OH��2CO3 2Cu+4CO��+H20����
2Cu+4CO��+H20����
��3������Zn��H2 �Ĺ�ϵʽ��n��Zn��=n��H2 ��=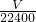 mol����ͭ��п�������ǣ�
mol����ͭ��п�������ǣ�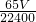 g��ͭ������Ϊ��w-
g��ͭ������Ϊ��w-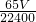 ��g����n��Cu��=
��g����n��Cu��=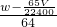 ��mol����
��mol����
������Ʒ��ͭ��п�����ʵ���֮���ǣ���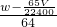 mol��
mol��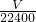 mol=
mol=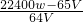 ��
��
�ʴ�Ϊ��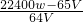 ��
��
��������1����װ�ÿ����������ȷֽ�����װ�ã���ʽ̼��ͭ�ֽ���Ҫ���ȣ��õ��ƾ��ƣ�
��2����Ӧ����̼�ͼ�ʽ̼��ͭ��������ͭ��һ��������ˮ������ʽ̼��ͭ���ϼ۱仯�������壬���ݻ��ϼ���������ƽ��
��3���ȸ���������������п�����ʵ�����Ȼ�����û�ͭ����w��������ʵ���֮�ȣ�
���������⿼���˼�ʽ̼��ͭ�����ʣ��漰�˻�ѧ����ʽ����д����Ӧװ��ѡ���֪ʶ�������Ѷ��еȣ�
�ʴ�Ϊ�������ƾ��ƣ�
��2���Ѽ�ʽ̼��ͭ��C��CuԪ�ػ��ϼ۱仯�������壬���ϼ��ܽ��ͣ�2����2-0��+��4-2��=6�ۣ�̼���ʻ��ϼ�����2�ۣ���̼�ļ�������3����ʽ̼��ͭ�Ļ�ѧ��������1
��ƽ��Ӧ����ʽ��3C+Cu2��OH��2CO3
 2Cu+4CO��+H20����
2Cu+4CO��+H20�����ʴ�Ϊ��4C+Cu2��OH��2CO3
 2Cu+4CO��+H20����
2Cu+4CO��+H20������3������Zn��H2 �Ĺ�ϵʽ��n��Zn��=n��H2 ��=
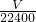 mol����ͭ��п�������ǣ�
mol����ͭ��п�������ǣ�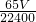 g��ͭ������Ϊ��w-
g��ͭ������Ϊ��w-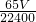 ��g����n��Cu��=
��g����n��Cu��=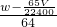 ��mol����
��mol����������Ʒ��ͭ��п�����ʵ���֮���ǣ���
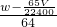 mol��
mol��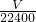 mol=
mol=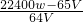 ��
���ʴ�Ϊ��
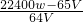 ��
����������1����װ�ÿ����������ȷֽ�����װ�ã���ʽ̼��ͭ�ֽ���Ҫ���ȣ��õ��ƾ��ƣ�
��2����Ӧ����̼�ͼ�ʽ̼��ͭ��������ͭ��һ��������ˮ������ʽ̼��ͭ���ϼ۱仯�������壬���ݻ��ϼ���������ƽ��
��3���ȸ���������������п�����ʵ�����Ȼ�����û�ͭ����w��������ʵ���֮�ȣ�
���������⿼���˼�ʽ̼��ͭ�����ʣ��漰�˻�ѧ����ʽ����д����Ӧװ��ѡ���֪ʶ�������Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ����ͭ���������������г��õĽ�����
����ͭ���������������г��õĽ�����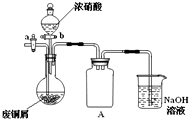 ��ʽ̼��ͭ[Cu2��OH��2CO3]��һ����;�㷺�Ļ���ԭ�ϣ�ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£�
��ʽ̼��ͭ[Cu2��OH��2CO3]��һ����;�㷺�Ļ���ԭ�ϣ�ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£�