��Ŀ����
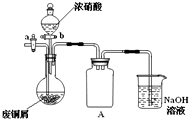 ��ʽ̼��ͭ[Cu2��OH��2CO3]��һ����;�㷺�Ļ���ԭ�ϣ�ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£�
��ʽ̼��ͭ[Cu2��OH��2CO3]��һ����;�㷺�Ļ���ԭ�ϣ�ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£���ͭм������ͭ
��ͼ���г�������ʡ�ԣ�����Ũ���Ỻ���ӵ���ͭм�У���ͭм����������ַ�Ӧ����ˣ��õ�����ͭ��Һ��
��ʽ̼��ͭ���Ʊ�
������Թ��м���̼������Һ������ͭ��Һ
��ˮԡ������70������
����0.4mol/L��NaOH ��Һ����pH��8.5�������á�����
������ˮϴ�ӣ���ɣ��õ���ʽ̼��ͭ��Ʒ
�ش�
��1��Ũ������ͭ��Ӧ�����ӷ���ʽ
Cu+4H++2NO3-=Cu2++2NO2��+2H2O
Cu+4H++2NO3-=Cu2++2NO2��+2H2O
����2��װ��A��������
��ֹ����
��ֹ����
����3����֪��NO+NO2+2NaOH�T2NaNO2+H2O��2NO2+2NaOH�TNaNO3+NaNO2+H2O��NO���ܵ�����NaOH��Һ��Ӧ��ʵ�����ʱ����β�������ʹװ���е��ж����屻NaOH��Һ������գ�
�رջ���b������a��ͨ��һ��ʱ�����
�رջ���b������a��ͨ��һ��ʱ�����
����4���������ϴ�ӵ�Ŀ����
ϴȥ��ʽ̼��ͭ����������Na+��NO3-
ϴȥ��ʽ̼��ͭ����������Na+��NO3-
����5������۹��˺����Һ�п��ܺ���CO32-������CO32- �ķ�����
ȡ������Һ���Թ��У�����ϡ���ᣬ������������ͨ�����ʯ��ˮ����Һ����ǣ�˵����CO32-
ȡ������Һ���Թ��У�����ϡ���ᣬ������������ͨ�����ʯ��ˮ����Һ����ǣ�˵����CO32-
����6����ʵ��õ�2.36g ��Ʒ��ֻ��CuO ���ʣ���ȡ����Ʒ�������ֽ���ȫ�õ�1.74g ���壬����Ʒ�м�ʽ̼��ͭ������������
0.94��94%
0.94��94%
����������1��Ũ������ͭ��Ӧ��������ͭ���������������ˮ�����ӷ���ʽ��Ũ����д��������ʽ��
��2��ͭ��Ũ���ᷴӦ�У�Բ����ƿ��ѹǿ��Ѹ�ټ�С��������������Һ�����뷴Ӧװ�ã�����Aװ�þͿ��Է�ֹ������
��3���رջ���b������a��ͨ��һ��ʱ���������װ���е��ж����嵼������������Һ�У�������������Һ������գ�
��4��ϴ�ӿɳ�ȥ��ʽ̼��ͭ����������������ƣ�
��5������ϡ���ᣬ�����Ƿ��ж�����̼����������������Һ���Ƿ���̼������ӣ�
��6�����ʽ̼��ͭ����������Ϊx�����ݷ�ӦCu2��OH��2CO3
2CuO+H2O+CO2�������ó������������ʽ�������ʽ̼��ͭ������������
��2��ͭ��Ũ���ᷴӦ�У�Բ����ƿ��ѹǿ��Ѹ�ټ�С��������������Һ�����뷴Ӧװ�ã�����Aװ�þͿ��Է�ֹ������
��3���رջ���b������a��ͨ��һ��ʱ���������װ���е��ж����嵼������������Һ�У�������������Һ������գ�
��4��ϴ�ӿɳ�ȥ��ʽ̼��ͭ����������������ƣ�
��5������ϡ���ᣬ�����Ƿ��ж�����̼����������������Һ���Ƿ���̼������ӣ�
��6�����ʽ̼��ͭ����������Ϊx�����ݷ�ӦCu2��OH��2CO3
| ||
����⣺��1��Ũ������ͭ��Ӧ�����ӷ���ʽΪCu+4H++2NO3-=Cu2++2NO2��+2H2O��
�ʴ�Ϊ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
��2������ͭ��Ũ���ᷴӦ�У�Բ����ƿ��ѹǿ��Ѹ�ټ�С��������������Һ�����뷴Ӧװ�ã�����Aװ�þͿ��Է�ֹ������
�ʴ�Ϊ����ֹ������
��3��ͨ���رջ���b������a��ͨ��һ��ʱ��������������Խ�װ���е��ж����嵼������������Һ�У�������������Һ������գ�
�ʴ�Ϊ���رջ���b������a��ͨ��һ��ʱ�������
��4��ͨ��ϴ�ӿɳ�ȥ��ʽ̼��ͭ����������������ƣ��ʴ�Ϊ��ϴȥ��ʽ̼��ͭ����������Na+��NO3-��
��5������۹��˺����Һ�п��ܺ���CO32-������CO32-�ķ���Ϊ��ȡ������Һ���Թ��У�����ϡ���ᣬ������������ͨ�����ʯ��ˮ����Һ����ǣ�˵����CO32-��
�ʴ�Ϊ��ȡ������Һ���Թ��У�����ϡ���ᣬ������������ͨ�����ʯ��ˮ����Һ����ǣ�˵����CO32-��
��6����ʽ̼��ͭ��ȫ�ֽ�õ�CuO������Ʒ�м�ʽ̼��ͭ����������Ϊx��
���ݼ�ʽ̼��ͭ�ķ���ʽ��Cu2��OH��2CO3
2CuO+H2O+CO2�� �������m
222 18 44 ��18+44��=62
2.36x ��2.36g-1.74g��
�ɵ�
=
��
���x=0.94��
�ʴ�Ϊ��0.94��94%��
�ʴ�Ϊ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
��2������ͭ��Ũ���ᷴӦ�У�Բ����ƿ��ѹǿ��Ѹ�ټ�С��������������Һ�����뷴Ӧװ�ã�����Aװ�þͿ��Է�ֹ������
�ʴ�Ϊ����ֹ������
��3��ͨ���رջ���b������a��ͨ��һ��ʱ��������������Խ�װ���е��ж����嵼������������Һ�У�������������Һ������գ�
�ʴ�Ϊ���رջ���b������a��ͨ��һ��ʱ�������
��4��ͨ��ϴ�ӿɳ�ȥ��ʽ̼��ͭ����������������ƣ��ʴ�Ϊ��ϴȥ��ʽ̼��ͭ����������Na+��NO3-��
��5������۹��˺����Һ�п��ܺ���CO32-������CO32-�ķ���Ϊ��ȡ������Һ���Թ��У�����ϡ���ᣬ������������ͨ�����ʯ��ˮ����Һ����ǣ�˵����CO32-��
�ʴ�Ϊ��ȡ������Һ���Թ��У�����ϡ���ᣬ������������ͨ�����ʯ��ˮ����Һ����ǣ�˵����CO32-��
��6����ʽ̼��ͭ��ȫ�ֽ�õ�CuO������Ʒ�м�ʽ̼��ͭ����������Ϊx��
���ݼ�ʽ̼��ͭ�ķ���ʽ��Cu2��OH��2CO3
| ||
222 18 44 ��18+44��=62
2.36x ��2.36g-1.74g��
�ɵ�
| 222 |
| 62 |
| 2.36x |
| (2.36-1.74) |
���x=0.94��
�ʴ�Ϊ��0.94��94%��
���������������ȡ��ʽ̼��ͭ�Ʒ����������ӷ���ʽ��д���������������֪ʶ����ֿ�����ѧ���ķ��������⡢������������һ����������Ŀ�������Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ����ͭ���������������г��õĽ�����
����ͭ���������������г��õĽ�����