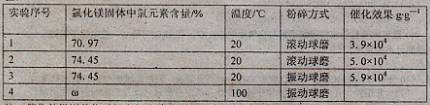
��Ŀ����
��12�֣���������ʵ��װ��ͼ�ش�װ���ô��ű�ʾ��
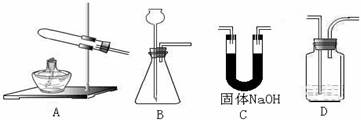
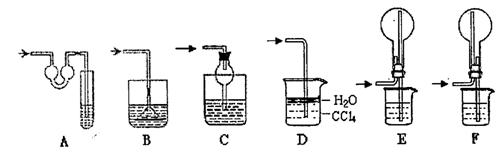
��ʵ����������ʱ��Ӧѡ�õķ���װ���� ��������ʱӦѡ�õķ���װ���� ���ƶ�����̼ʱӦѡ�õķ���װ���� �����������̼�����ѡ��Dװ�ã���װ����ʢ�ŵ��Լ�һ���� ��
����п����Ũ���ᷴӦ�����������Ƴɵ�����ͨ�����������а�ɫ������˵�������к��У�д��ѧʽ�� ����Ӧ����ʽΪ ����Ҫ�Ƴ�������������������װ���Ӧѡ�õ�һ��װ���� ����װ����ҩƷ�������� ��

��ʵ����������ʱ��Ӧѡ�õķ���װ���� ��������ʱӦѡ�õķ���װ���� ���ƶ�����̼ʱӦѡ�õķ���װ���� �����������̼�����ѡ��Dװ�ã���װ����ʢ�ŵ��Լ�һ���� ��
����п����Ũ���ᷴӦ�����������Ƴɵ�����ͨ�����������а�ɫ������˵�������к��У�д��ѧʽ�� ����Ӧ����ʽΪ ����Ҫ�Ƴ�������������������װ���Ӧѡ�õ�һ��װ���� ����װ����ҩƷ�������� ��
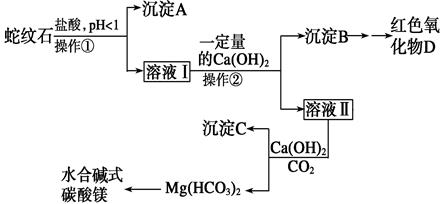
��1��A��B�� B������ʯ��ˮ��2��HCl ����ȥHCl������H2
��

��ϰ��ϵ�д�
�����Ŀ
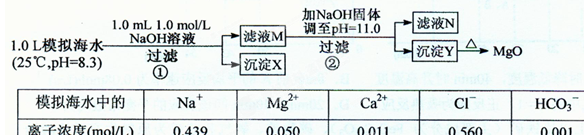
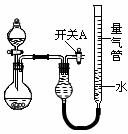
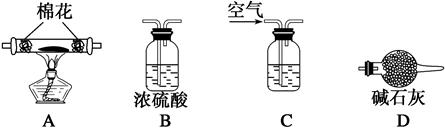
 3��ʵ�����ʣ���NH3�����մ��������¸���β������װ���У��ʺ�������NH3�������ܷ�ֹ��������
3��ʵ�����ʣ���NH3�����մ��������¸���β������װ���У��ʺ�������NH3�������ܷ�ֹ�������� 

 �ȣ�����������Ĺ�ҵ���������ۺ����öԻ�������������ʵ���塣���������������Ʊ�����Ȳ�Ʒ��ʵ���������£�
�ȣ�����������Ĺ�ҵ���������ۺ����öԻ�������������ʵ���塣���������������Ʊ�����Ȳ�Ʒ��ʵ���������£�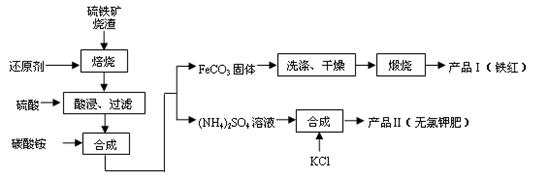
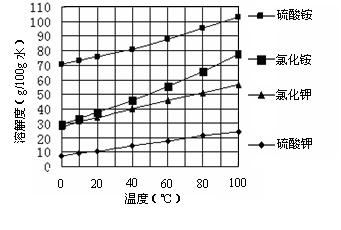
 Һ���ж�Ba(NO3)2�ѹ����ķ����� ��
Һ���ж�Ba(NO3)2�ѹ����ķ����� ��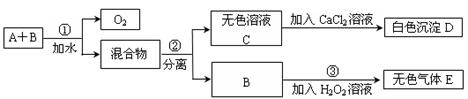
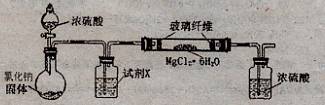
 ���ˣ�
���ˣ�