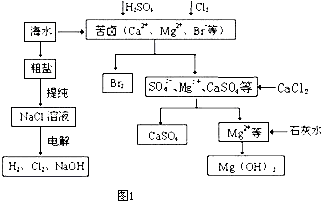
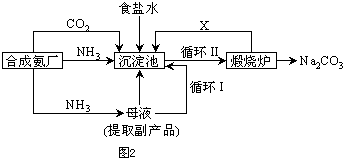
��Ŀ����
NH3��CO����Ҫ�Ļ���ԭ������
��1��һ�������£�CO��H2��Ӧ���Ƶü״�CO+2H2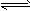 CH3OH
CH3OH
ͼ1��ʾ�÷�Ӧ���й����е������仯������aδʹ�ô���������bʹ�ô�������ͼ2��ʾһ���¶��£������Ϊ2L���ܱ������м���4mol H2��һ������CO��CO��CH3OH(g)��Ũ����ʱ��仯�����
��1��һ�������£�CO��H2��Ӧ���Ƶü״�CO+2H2
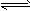 CH3OH
CH3OH ͼ1��ʾ�÷�Ӧ���й����е������仯������aδʹ�ô���������bʹ�ô�������ͼ2��ʾһ���¶��£������Ϊ2L���ܱ������м���4mol H2��һ������CO��CO��CH3OH(g)��Ũ����ʱ��仯�����

�ٸ�����ͼ1��д���ڸ�������CO�Ʊ��״����Ȼ�ѧ��Ӧ����ʽ__________________________
�ڸ�����ͼ2����ø��¶��£���ӦCO(g)+2H2(g) CH3OH(g)��ƽ�ⳣ��Ϊ_________________
CH3OH(g)��ƽ�ⳣ��Ϊ_________________
��ͼ2��ʾ�ľ��巴Ӧ�������¶ȱ��ֲ��䡢��ѹ����£�����ɱ䣩�ﵽƽ�⣬��H2��ƽ��ת���ʽ�
__________ �����������С�����䡱����
��2����֪��
a�������£�CH3COOH��NH3��H2O�ĵ���ƽ�ⳣ����Ϊ1��74��10-5
b��CH3COOH + NaHCO3��CH3COONa+ CO2��+ H2O
��NH4HCO3��Һ��______�ԣ���ᡱ��������С�������Һ��Ũ������������______�������
_______�������ѧʽ����
��3�������£���һ������ϡ��ˮ����μ���Ũ����ͬ��ϡ���ᣬֱ�����������
�ٵ���Һ������Ũ�ȹ�ϵ����c(NH4+)<c(Cl-)ʱ����Ӧ���������Ϊ_____________
A�� ����㣬��ˮʣ�� B�� ��ˮ������ǡ����ȫ��Ӧ C�� �������
��ʵ������У�H2O�ĵ���̶���_______��________�����������С�����䡱����
�ڸ�����ͼ2����ø��¶��£���ӦCO(g)+2H2(g)
 CH3OH(g)��ƽ�ⳣ��Ϊ_________________
CH3OH(g)��ƽ�ⳣ��Ϊ_________________ ��ͼ2��ʾ�ľ��巴Ӧ�������¶ȱ��ֲ��䡢��ѹ����£�����ɱ䣩�ﵽƽ�⣬��H2��ƽ��ת���ʽ�
__________ �����������С�����䡱����
��2����֪��
a�������£�CH3COOH��NH3��H2O�ĵ���ƽ�ⳣ����Ϊ1��74��10-5
b��CH3COOH + NaHCO3��CH3COONa+ CO2��+ H2O
��NH4HCO3��Һ��______�ԣ���ᡱ��������С�������Һ��Ũ������������______�������
_______�������ѧʽ����
��3�������£���һ������ϡ��ˮ����μ���Ũ����ͬ��ϡ���ᣬֱ�����������
�ٵ���Һ������Ũ�ȹ�ϵ����c(NH4+)<c(Cl-)ʱ����Ӧ���������Ϊ_____________
A�� ����㣬��ˮʣ�� B�� ��ˮ������ǡ����ȫ��Ӧ C�� �������
��ʵ������У�H2O�ĵ���̶���_______��________�����������С�����䡱����
��1����CO(g)+2H2(g)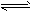 CH3OH(g) ��H=��91kJ��mol-1����12L2/mol2��������
CH3OH(g) ��H=��91kJ��mol-1����12L2/mol2��������
��2�����ԣ�NH4+��HCO3-
��3����ABC��������С
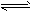 CH3OH(g) ��H=��91kJ��mol-1����12L2/mol2��������
CH3OH(g) ��H=��91kJ��mol-1����12L2/mol2����������2�����ԣ�NH4+��HCO3-
��3����ABC��������С

��ϰ��ϵ�д�
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
�����Ŀ
 I������������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ���±�1�dz����¼�������ĵ���ƽ�ⳣ����Ka��������ĵ���ƽ�ⳣ����Kb����
I������������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ���±�1�dz����¼�������ĵ���ƽ�ⳣ����Ka��������ĵ���ƽ�ⳣ����Kb����