��Ŀ����
A��E����ѧ������5�ֻ����A��B�����������֮���ת����ϵ��ͼ��ʾ��
������˵����ȷ
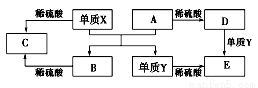
A������X��A��Ӧ�Ļ�ѧ����ʽ��Al2O3+2Fe Fe2O3+2Al
Fe2O3+2Al
B������D��Һ�н��������ӵķ�Ӧ��Fe3++3SCN-=Fe(SCN)3��
C������Y��һ������������ˮ�����û���Ӧ
D�����ڻ�����B��C���������ᷴӦ��������Ӧ���������߾������Ի�����
��ϰ��ϵ�д�
�����Ŀ
6��Ԫ�ص�ԭ�ӽṹ���������ʺ������ڱ��е�λ�ã�����˵����ȷ���ǣ�������
| A�� | Ԫ��ԭ�ӵ���������������Ԫ�ص�����ϼ� | |
| B�� | �����ԭ���У�����ԭ�Ӻ˽Ͻ����������˶��ĵ��������ϸ� | |
| C�� | P��S��Cl�ǽ����Ժ�����������Ӧ��ˮ��������Ծ�������ǿ | |
| D�� | Ԫ�����ڱ���λ�ڽ����ͷǽ����ֽ��߸�����Ԫ�����ڹ���Ԫ�� |
4�� 2015��2�������о���Ա��CO��O������һ���ɴ������棬�ü������彫����ȵ�2000K���ɹ��۲쵽CO��O�γɻ�ѧ������CO2��ȫ���̣�����˵����ȷ���ǣ�������
2015��2�������о���Ա��CO��O������һ���ɴ������棬�ü������彫����ȵ�2000K���ɹ��۲쵽CO��O�γɻ�ѧ������CO2��ȫ���̣�����˵����ȷ���ǣ�������
 2015��2�������о���Ա��CO��O������һ���ɴ������棬�ü������彫����ȵ�2000K���ɹ��۲쵽CO��O�γɻ�ѧ������CO2��ȫ���̣�����˵����ȷ���ǣ�������
2015��2�������о���Ա��CO��O������һ���ɴ������棬�ü������彫����ȵ�2000K���ɹ��۲쵽CO��O�γɻ�ѧ������CO2��ȫ���̣�����˵����ȷ���ǣ�������| A�� | CO��CO2���������������� | |
| B�� | �γɻ�ѧ��ʱ���������� | |
| C�� | �ɴ������Ըı�÷�Ӧ���ʱ� | |
| D�� | CO��O�γɻ�ѧ���������е���ת�� |
19������8����
��CH2�TCH2�� ��CH3OH����CH3Cl����CCl4��HCOOCH3����CH3COOCH2CH3����CH3COOH�������ŵIJ�ͬ�ɷ�Ϊ��������
��CH3OH����CH3Cl����CCl4��HCOOCH3����CH3COOCH2CH3����CH3COOH�������ŵIJ�ͬ�ɷ�Ϊ��������
��CH2�TCH2��
 ��CH3OH����CH3Cl����CCl4��HCOOCH3����CH3COOCH2CH3����CH3COOH�������ŵIJ�ͬ�ɷ�Ϊ��������
��CH3OH����CH3Cl����CCl4��HCOOCH3����CH3COOCH2CH3����CH3COOH�������ŵIJ�ͬ�ɷ�Ϊ��������| A�� | 4�� | B�� | 5�� | C�� | 6�� | D�� | 8�� |
2����ͼ��ʾ��ʵ�飬�ܴﵽʵ��Ŀ���ǣ�������
| A | B | C | D |
 | 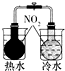 | 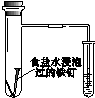 | 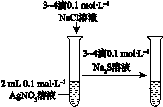 |
| ��֤��ѧ�� ת��Ϊ���� | ֤���¶� ��ƽ���ƶ���Ӱ�� | ��֤���������ⸯʴ | ��֤AgCl �ܽ�ȴ���Ag2S |
| A�� | A | B�� | B | C�� | C | D�� | D |
3��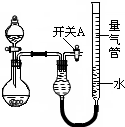 ijͬѧ�����ͼ��ʾװ�÷ֱ����̽��ʵ�飨�г�װ������ȥ��
ijͬѧ�����ͼ��ʾװ�÷ֱ����̽��ʵ�飨�г�װ������ȥ��
��ش��������⣺
��1��Cu��ϡHNO3��Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��2����ͬѧ��Ϊʵ��ٿ�ͨ���ռ�����NO������������̽��Cu��Ʒ�Ĵ��ȣ�����Ϊ�Ƿ���У������У����С������У��������ԭ����ΪNO����װ���п�����Ӧ������ˮ��ʹ��õ�NO�����������
��3��ʵ��ڡ����У��������е�Һ����÷ֱ���A��C����ֻѡһ�֣�
A��CCl4 B��H2OC������NaHCO3�� D��Һ����Na2CO3��Һ
��4����ʵ��Ӧ�������ܶ�ζ���������ʱӦע�⣺
�ٻָ������£���ʹ����������Һ���࣬�������밼Һ����ʹ���ƽ��
��5��ʵ������ڲⶨ�Ͻ���������������������������ݣ�����������ѻ���ɱ�״����
���������������ݼ���þ���Ͻ���������������27%��
 ijͬѧ�����ͼ��ʾװ�÷ֱ����̽��ʵ�飨�г�װ������ȥ��
ijͬѧ�����ͼ��ʾװ�÷ֱ����̽��ʵ�飨�г�װ������ȥ��| ʵ�� | ҩƷ | ��ȡ���� | �������е�Һ�� |
| �� | Cu��ϡHNO3 | H2O | |
| �� | NaOH���塢Ũ��ˮ | NH3 | |
| �� | Na2CO3���塢ϡH2SO4 | CO2 | |
| �� | þ���Ͻ�NaOH��Һ�������� | H2 | H2O |
��1��Cu��ϡHNO3��Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��2����ͬѧ��Ϊʵ��ٿ�ͨ���ռ�����NO������������̽��Cu��Ʒ�Ĵ��ȣ�����Ϊ�Ƿ���У������У����С������У��������ԭ����ΪNO����װ���п�����Ӧ������ˮ��ʹ��õ�NO�����������
��3��ʵ��ڡ����У��������е�Һ����÷ֱ���A��C����ֻѡһ�֣�
A��CCl4 B��H2OC������NaHCO3�� D��Һ����Na2CO3��Һ
��4����ʵ��Ӧ�������ܶ�ζ���������ʱӦע�⣺
�ٻָ������£���ʹ����������Һ���࣬�������밼Һ����ʹ���ƽ��
��5��ʵ������ڲⶨ�Ͻ���������������������������ݣ�����������ѻ���ɱ�״����
| ��� | þ���Ͻ����� | �����ܵ�һ�ζ��� | �����ܵڶ��ζ��� |
| 1 | 1.0g | 10.0mL | 347.5mL |
| 2 | 1.0g | 10.0mL | 335.0mL |
| 3 | 1.0g | 10.0mL | 344.5mL |


 ���������������е�����Ԫ����
���������������е�����Ԫ���� ��
�� 2��3��4-����-3-�һ�����
2��3��4-����-3-�һ����� 4-��-2-��ϩ
4-��-2-��ϩ �ұ�
�ұ�


 ��
��