ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩIΓΔ≈δ÷Τ960 mL 0.3mol/L NaOH»ή“ΚΓΘΨΏΧε»γœ¬ΘΚ
Θ®1Θ©–η__________________________g NaOHΓΘ
Θ®2Θ©≈δ÷ΤΙΐ≥Χ÷–,œ¬Ν–“«Τς≤ΜΜα”ΟΒΫΒΡ «________________Θ§Θ®Χν–ρΚ≈Θ©…–»±…ΌΒΡ÷ς“Σ≤ΘΝß“«Τς «_________ΓΘ
AΘ°Ά–≈ΧΧλΤΫ BΘ°250mL»ίΝΩΤΩ CΘ°≤ΘΝßΑτ DΘ°ΫΚΆΖΒΈΙή
IIΓΔ““Ά§―ß”Ο÷ ΝΩΖ÷ ΐΈΣ98%Θ§ΟήΕ»ΈΣ1.84g/cm3ΒΡ≈®ΝρΥα≈δ÷Τ90mL 2.3 mol/LΒΡœΓΝρΥαΓΘΆ®ΙΐΦΤΥψΘ§”ΟΝΩΆ≤ΝΩ»Γ_________ml ≈®ΝρΥαΘ§―Γ”ΟΒΡΝΩΆ≤________ΓΘΘ®―ΓΧν15mLΘ§25mLΘ§ 50mLΘ§ 100mLΘ©
IIIΓΔœ¬Ν–≤ΌΉςΜα Ι≈δ÷ΤΒΡ»ή“Κ≈®Ε»ΤΪΒΆΒΡ «___________(ΧνΉ÷ΡΗ)ΓΘ
AΓΔ≈δ÷Τ«β―θΜ·ΡΤ»ή“Κ ±Θ§≥Τ»Γ“―Έϋ≥±ΒΡ«β―θΜ·ΡΤΙΧΧε
BΓΔ≈δ÷Τ«β―θΜ·ΡΤ»ή“Κ ±Θ§»ίΝΩΤΩ÷–”–…ΌΝΩΥ°
CΓΔΖΔœ÷»ή“Κ“ΚΟφ≥§ΙΐΩΧΕ»œΏΘ§”ΟΈϋΙήΈϋ≥ω…ΌΝΩΥ°Θ§ Ι“ΚΟφΫΒ÷ΝΩΧΕ»œΏ
DΓΔΕ®»ί ±Η© ”»ίΝΩΤΩΩΧΕ»œΏ
ΓΨ¥πΑΗΓΩ12.0 B 1000mL»ίΝΩΤΩ 11.3 15mL AC
ΓΨΫβΈωΓΩ
IΓΔΘ®1Θ©960 mL 0.3mol/L NaOH»ή“Κ÷ΜΡή Ι”Ο1000mLΒΡ»ίΝΩΤΩΘ§0.3mol/L ΓΝ1L=0.3mol Ι –η“Σ«β―θΜ·ΡΤΒΡ÷ ΝΩΈΣm[NaOH]=0.3ΓΝ(23+16+1)=12gΘ§
Ι ¥πΑΗΈΣ 12gΘΜ
Θ®2Θ©≈δ÷Τ960 mL 0.3mol/L NaOH»ή“ΚΒΡΙΐ≥Χ÷–Θ§≤ΜΜα”ΟΒΫΒΡ «250mLΒΡ»ίΝΩΤΩΘ§ΜΙ»±…Ό÷ς“Σ≤ΘΝß“«Τς «1000mLΒΡ»ίΝΩΤΩΓΘ
Δρ.÷ ΝΩΖ÷ ΐΈΣ98%Θ§ΟήΕ»ΈΣ1.84g/cm3ΒΡ≈®ΝρΥαΒΡΝΩ≈®Ε»ΈΣΘΚ![]() Θ§œΓ Ά«ΑΚσΝρΥαΒΡΈο÷ ΒΡΝΩ≤Μ±δΘ§»ή“ΚœΓ ΆΕ®¬…C≈®V≈®=CœΓVœΓΘ§Ι VΘ®≈®Θ©=
Θ§œΓ Ά«ΑΚσΝρΥαΒΡΈο÷ ΒΡΝΩ≤Μ±δΘ§»ή“ΚœΓ ΆΕ®¬…C≈®V≈®=CœΓVœΓΘ§Ι VΘ®≈®Θ©= ![]() =11.25mLΓ÷11.3mLΓΘΙ ―Γ”Ο15mLΒΡΝΩΆ≤ΗϋΈΣΨΪ»ΖΓΘ
=11.25mLΓ÷11.3mLΓΘΙ ―Γ”Ο15mLΒΡΝΩΆ≤ΗϋΈΣΨΪ»ΖΓΘ
Δσ.AΓΔ≈δ÷Τ«β―θΜ·ΡΤ»ή“Κ ±Θ§≥Τ»Γ“―Έϋ≥±ΒΡ«β―θΜ·ΡΤΙΧΧεΜα Ι ΒΦ ΒΡ«β―θΜ·ΡΤΒΡΈο÷ ΒΡΝΩΤΪΒΆΘ§Ι ≈δ÷ΟΒΡ»ή“Κ≈®Ε»ΤΪΒΆΘ§Ι A―Γœν’ΐ»ΖΘΜ
BΓΔ≈δ÷Τ«β―θΜ·ΡΤ»ή“Κ ±Θ§»ίΝΩΤΩ÷–”–…ΌΝΩΥ°Ε‘ Β―ιΫαΙϊΈό”ΑœλΘ§Ι B―Γœν¥μΈσΘΜ
CΓΔΖΔœ÷»ή“Κ“ΚΟφ≥§ΙΐΩΧΕ»œΏΘ§”ΟΈϋΙήΈϋ≥ω…ΌΝΩΥ°Θ§Μα Ι»ή÷ ÷ ΝΩΦθ…ΌΘ§Ι C―Γœν’ΐ»ΖΘΜ
DΓΔΕ®»ί ±Η© ”»ίΝΩΤΩΩΧΕ»œΏΜα ΙΦ”Υ°ΧεΜΐΤΪ–ΓΘ§»ή“Κ≈®Ε»ΤΪ¥σΘ§Ι D―Γœν¥μΈσΘΜ
Ι ¥πΑΗΈΣBΘΜ1000mL»ίΝΩΤΩΘΜ11.3ΘΜ15mLΘΜACΓΘ

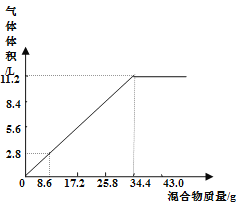
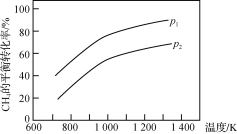
ΓΨΧβΡΩΓΩ““θΘ±ΫΑΖ(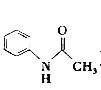 )‘ΎΙΛ“Β…œΩ…”ΟΉςœπΫΚΝρΜ·¥ΌΫχΦΝΓΔœΥΈ§θΞΆΩΝœΒΡΈ»Ε®ΦΝΓΔΙΐ―θΜ·«βΒΡΈ»Ε®ΦΝΒ»Θ§Ω…Ά®Ιΐ±ΫΑΖ(
)‘ΎΙΛ“Β…œΩ…”ΟΉςœπΫΚΝρΜ·¥ΌΫχΦΝΓΔœΥΈ§θΞΆΩΝœΒΡΈ»Ε®ΦΝΓΔΙΐ―θΜ·«βΒΡΈ»Ε®ΦΝΒ»Θ§Ω…Ά®Ιΐ±ΫΑΖ(![]() )ΚΆ““Υατϊ(
)ΚΆ““Υατϊ(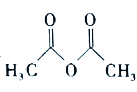 )Ζ¥”Π÷ΤΒΟΓΘ“―÷ΣΘΚ¥Ω““θΘ±ΫΑΖ «ΑΉ…ΪΤ§Ή¥ΨßΧεΘ§œύΕ‘Ζ÷Ή”÷ ΝΩΈΣ135Θ§»έΒψΈΣ
)Ζ¥”Π÷ΤΒΟΓΘ“―÷ΣΘΚ¥Ω““θΘ±ΫΑΖ «ΑΉ…ΪΤ§Ή¥ΨßΧεΘ§œύΕ‘Ζ÷Ή”÷ ΝΩΈΣ135Θ§»έΒψΈΣ![]() Θ§“Ή»ή”Ύ”–Μζ»ήΦΝΓΘ‘ΎΥ°÷–ΒΡ»ήΫβΕ»»γœ¬ΓΘ
Θ§“Ή»ή”Ύ”–Μζ»ήΦΝΓΘ‘ΎΥ°÷–ΒΡ»ήΫβΕ»»γœ¬ΓΘ
Έ¬Ε»/Γφ | 20 | 25 | 50 | 80 | 100 |
»ήΫβΕ»/( | 0.46 | 0.56 | 0.84 | 3.45 | 5.5 |
Β―ι “÷Τ±Η““θΘ±ΫΑΖΒΡ≤Ϋ÷η»γœ¬(≤ΩΖ÷ΉΑ÷Ο Γ¬‘)ΘΚ
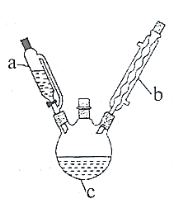
Δώ.¥÷““θΘ±ΫΑΖΒΡ÷Τ±ΗΓΘΫΪ![]() ““ΥατϊΖ≈»κ»ΐΩΎ…’ΤΩ
““ΥατϊΖ≈»κ»ΐΩΎ…’ΤΩ![]() ÷–Θ§‘Ύ
÷–Θ§‘Ύ![]() ÷–Ζ≈»κ
÷–Ζ≈»κ![]() –¬÷ΤΒΟΒΡ±ΫΑΖΓΘΫΪ±ΫΑΖ‘Ύ “Έ¬œ¬÷πΒΈΒΈΦ”ΒΫ»ΐΩΎ…’ΤΩ÷–ΓΘ±ΫΑΖΒΈΦ”Άξ±œΘ§‘Ύ ·ΟόΆχ…œ”Ο–ΓΜπΦ”»»ΜΊΝς
–¬÷ΤΒΟΒΡ±ΫΑΖΓΘΫΪ±ΫΑΖ‘Ύ “Έ¬œ¬÷πΒΈΒΈΦ”ΒΫ»ΐΩΎ…’ΤΩ÷–ΓΘ±ΫΑΖΒΈΦ”Άξ±œΘ§‘Ύ ·ΟόΆχ…œ”Ο–ΓΜπΦ”»»ΜΊΝς![]() Θ§ Ι÷°≥δΖ÷Ζ¥”ΠΓΘ¥ΐΖ¥”ΠΆξ≥…Θ§‘Ύ≤ΜΕœΫΝΑηœ¬Θ§≥Ο»»Α―Ζ¥”ΠΜλΚœΈοΜΚ¬ΐΒΊΒΙ»κ Δ”–
Θ§ Ι÷°≥δΖ÷Ζ¥”ΠΓΘ¥ΐΖ¥”ΠΆξ≥…Θ§‘Ύ≤ΜΕœΫΝΑηœ¬Θ§≥Ο»»Α―Ζ¥”ΠΜλΚœΈοΜΚ¬ΐΒΊΒΙ»κ Δ”–![]() άδΥ°ΒΡ…’±≠÷–Θ§““θΘ±ΫΑΖΨßΧεΈω≥ωΓΘ≥δΖ÷ά以÷Ν “Έ¬ΚσΘ§Φθ―ΙΙΐ¬ΥΘ§”Ο______œ¥Β”ΨßΧε2-3¥ΈΓΘ”Ο¬Υ“Κ≥εœ¥…’±≠…œ≤–ΝτΒΡΨßΧεΘ§‘Ό¥ΈΙΐ¬ΥΘ§ΝΫ¥ΈΙΐ¬ΥΒΟΒΫΒΡΙΧΧεΚœ≤Δ‘Ύ“ΜΤπΓΘ
άδΥ°ΒΡ…’±≠÷–Θ§““θΘ±ΫΑΖΨßΧεΈω≥ωΓΘ≥δΖ÷ά以÷Ν “Έ¬ΚσΘ§Φθ―ΙΙΐ¬ΥΘ§”Ο______œ¥Β”ΨßΧε2-3¥ΈΓΘ”Ο¬Υ“Κ≥εœ¥…’±≠…œ≤–ΝτΒΡΨßΧεΘ§‘Ό¥ΈΙΐ¬ΥΘ§ΝΫ¥ΈΙΐ¬ΥΒΟΒΫΒΡΙΧΧεΚœ≤Δ‘Ύ“ΜΤπΓΘ
Δρ.““θΘ±ΫΑΖΒΡΧα¥ΩΓΘΫΪ…œ ω÷ΤΒΟΒΡ¥÷““θΘ±ΫΑΖΙΧΧε“Τ»κ![]() …’±≠÷–Θ§Φ”»κ
…’±≠÷–Θ§Φ”»κ![]() »»Υ°Θ§Φ”»»÷ΝΖ–ΧΎΘ§¥ΐ¥÷““θΘ±ΫΑΖΆξ»Ϊ»ήΫβΚσΘ§‘Ό≤ΙΦ”…ΌΝΩ’τΝσΥ°ΓΘ…‘άδΚσΘ§Φ”»κ…ΌΝΩΜν–‘ΧΩΈϋΗΫ…ΪΥΊΒ»‘”÷ Θ§‘ΎΫΝΑηœ¬ΈΔΖ–
»»Υ°Θ§Φ”»»÷ΝΖ–ΧΎΘ§¥ΐ¥÷““θΘ±ΫΑΖΆξ»Ϊ»ήΫβΚσΘ§‘Ό≤ΙΦ”…ΌΝΩ’τΝσΥ°ΓΘ…‘άδΚσΘ§Φ”»κ…ΌΝΩΜν–‘ΧΩΈϋΗΫ…ΪΥΊΒ»‘”÷ Θ§‘ΎΫΝΑηœ¬ΈΔΖ–![]() Θ§≥Ο»»Ιΐ¬ΥΓΘ¥ΐ¬Υ“Κά以÷Ν “Έ¬Θ§”–ΨßΧεΈω≥ωΘ§______ΓΔ______Θ§Η…‘οΚσ≥ΤΝΩ≤ζΤΖΈΣ
Θ§≥Ο»»Ιΐ¬ΥΓΘ¥ΐ¬Υ“Κά以÷Ν “Έ¬Θ§”–ΨßΧεΈω≥ωΘ§______ΓΔ______Θ§Η…‘οΚσ≥ΤΝΩ≤ζΤΖΈΣ![]() ΓΘ
ΓΘ
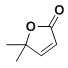
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)“«Τς![]() ΒΡΟϊ≥Τ «______________ΓΘ
ΒΡΟϊ≥Τ «______________ΓΘ
(2)–¥≥ω÷Τ±Η““θΘ±ΫΑΖΒΡΜ·―ßΖΫ≥Χ Ϋ______________ΓΘ
(3)≤ΫΨέI÷–Θ§Φθ―ΙΙΐ¬ΥΒΡ”≈Βψ «_____ΘΜ”Ο¬Υ“ΚΕχ≤Μ”Ο’τΝσΥ°≥εœ¥…’±≠ΒΡ‘≠“ρ «_________ΘΜœ¥Β”ΨßΧεΉνΚΟ―Γ”Ο________(ΧνΉ÷ΡΗ)ΓΘ
A.““¥Φ B.![]() C.άδΥ° D.““Ο―
C.άδΥ° D.““Ο―
(4)≤Ϋ÷ηΔρ÷–Θ§¥÷““θΘ±ΫΑΖ»ήΫβΚσΘ§≤ΙΦ”…ΌΝΩ’τΝσΥ°ΒΡΡΩΒΡ «______________ΓΘ
(5)≤Ϋ÷ηΔρ÷–Θ§Η…‘ο«ΑΒΡ≤ΌΉς «______________ΓΘ…œ ωΧα¥Ω““θΘ±ΫΑΖΒΡΖΫΖ®Ϋ–_____________ΓΘ
(6)““θΘ±ΫΑΖΒΡ≤ζ¬ ΈΣ______________ΓΘ(ΦΤΥψΫαΙϊ±ΘΝτ3ΈΜ”––ß ΐΉ÷)