ЬтФПФкШн
вбжЊЃК25ЁцЪБЃЌKa(HAc)=1.7ЁС10-5 mol /LЁЃЯжгаШчЯТШмвКЃК
Ђй0.1mol/LHAcгы0.1mol/LNaOHШмвКЕШЬхЛ§ЛьКЯвК
ЂкpH = 3ЕФHAcгыpH = 11ЕФNaOHШмвКЕШЬхЛ§ЛьКЯвК
Ђл0.1 mol /LH2SO4гы0.2mol/LNaOHШмвКЕШЬхЛ§ЛьКЯвК
ГЃЮТЪБЃЌШ§епpHДѓаЁЙиЯЕе§ШЗЕФЪЧ
AЃЎЂйЃОЂлЃОЂк BЃЎЂлЃОЂйЃОЂк CЃЎЂйЃОЂкЃОЂл DЃЎЂкЃОЂлЃОЂй
A
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКNaOHЪЧЧПМюЃЌHAcЪЧШѕЫсЁЃЂй0.1mol/LHAcгы0.1mol/LNaOHШмвКЕШЬхЛ§ЛьКЯвКЪБЧЁКУЭъШЋЗЂЩњЗДгІЕУЕНNaAcЁЃгЩгкNaAcЪЧЧПМюШѕЫсбЮЁЃдкШмвКжаAc-ЗЂЩњЫЎНтЗДгІЁЃAc-+H2O HAc+ OH-ЁЃЫљвдШмвКЯдМюадЁЃЂкpH = 3ЕФHAcШмвКc(H+)=10-3mol/L,гЩгкHAcЪЧШѕЫсЃЌВПЗжЕчРыЃЌЫљвдc(HAc) ЃО10-3mol/L pH = 11ЕФNaOHШмвК,c(OH-)=10-3mol/LЁЃШєЖўепЕШЬхЛ§ЛьКЯвКдђШмвКЮЊNaAcКЭHAcЕФЛьКЯШмвКЁЃгЩгкHAcЕФЕчРызїгУДѓгкAc-ЫЎНтзїгУЃЌЫљвдШмвКЯдЫсадЁЃЂл0.1 mol /LH2SO4гы0.2mol/LNaOH.вђЮЊH2SO4ЪЧЖўдЊЧПЫсЃЌNaOHЪЧвЛдЊЧПМюЃЌЫљвдСПШмвКжаЕФc(OH-)= c(H+)ШєЕШЬхЛ§ЛьКЯвКЃЌдђn(OH-)=n(H+).вђДЫШмвКЯджаадЁЃГЃЮТЪБЃЌШ§епpHДѓаЁЙиЯЕе§ШЗЕФЪЧЂйЃОЂлЃОЂкЁЃбЁЯюЮЊAЁЃ
HAc+ OH-ЁЃЫљвдШмвКЯдМюадЁЃЂкpH = 3ЕФHAcШмвКc(H+)=10-3mol/L,гЩгкHAcЪЧШѕЫсЃЌВПЗжЕчРыЃЌЫљвдc(HAc) ЃО10-3mol/L pH = 11ЕФNaOHШмвК,c(OH-)=10-3mol/LЁЃШєЖўепЕШЬхЛ§ЛьКЯвКдђШмвКЮЊNaAcКЭHAcЕФЛьКЯШмвКЁЃгЩгкHAcЕФЕчРызїгУДѓгкAc-ЫЎНтзїгУЃЌЫљвдШмвКЯдЫсадЁЃЂл0.1 mol /LH2SO4гы0.2mol/LNaOH.вђЮЊH2SO4ЪЧЖўдЊЧПЫсЃЌNaOHЪЧвЛдЊЧПМюЃЌЫљвдСПШмвКжаЕФc(OH-)= c(H+)ШєЕШЬхЛ§ЛьКЯвКЃЌдђn(OH-)=n(H+).вђДЫШмвКЯджаадЁЃГЃЮТЪБЃЌШ§епpHДѓаЁЙиЯЕе§ШЗЕФЪЧЂйЃОЂлЃОЂкЁЃбЁЯюЮЊAЁЃ
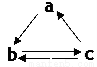
ПМЕуЃКПМВщЕШЬхЛ§ЫсЁЂМюШмвКЛьКЯЪБЕФpHДѓаЁЙиЯЕЕФжЊЪЖЁЃ

 ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ
ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИЯТБэЪЧдЊЫижмЦкБэЕФвЛВПЗж, еыЖдБэжаЕФЂйЁЋЂтжждЊЫи,ЬюаДЯТСаПеАз:
жїзх жмЦк | ЂёA | ЂђA | ЂѓA | ЂєA | ЂѕA | ЂіA | ЂїA | 0зх |
1 | Ђй |
|
| |||||
2 |
|
|
| Ђк | Ђл | Ђм |
|
|
3 | Ђн |
| Ђо |
| Ђп |
| Ђр | Ђс |
4 | Ђт |
|
|
|
|
|
|
|
ЃЈ1ЃЉаДгЩЩЯЪідЊЫизщГЩЕФЗжзгжаЕчзгзмЪ§ЮЊ10ЕФЗжзгЃЈжСЩйСНжжЃЉЃК ЁЃ
ЃЈ2ЃЉБШНЯЃКЂкЁЂЂлЁЂЂмЁЂЂнЕФЕквЛЕчРыФмЃК ЃО ЃО ЃО ЃЈаДдЊЫиЗћКХЃЉЁЃ
ЃЈ3ЃЉдкзюИпМлбѕЛЏЮяЕФЫЎЛЏЮяжаЃЌЫсадзюЧПЕФЛЏКЯЮяЕФЗжзгЪНЪЧ ЃЛМюадзюЧПЕФЛЏКЯЮяЕФЕчзгЪНЪЧ:
ЃЈ4ЃЉОпгаСНадЕФзюИпМлбѕЛЏЮяЪЧ (ЬюЛЏбЇЪН)ЃЛаДГіЦфгыЧтбѕЛЏФЦШмвКЗДгІЕФРызгЗНГЬЪНЃК ЁЃ
ЃЈ5ЃЉЯТБэЮЊдзгађЪ§вРДЮдіДѓЕФЖЬжмЦкдЊЫиAЁЋEЕФЕквЛЕНЕкЮхЕчРыФмЪ§ОнЁЃ
ЕчРыФмI(eV)AB C D E
I111ЃЎ313ЃЎ65ЃЎ27ЃЎ66ЃЎ0
I224ЃЎ435ЃЎ149ЃЎ315ЃЎ018ЃЎ8
I347ЃЎ954ЃЎ971ЃЎ680ЃЎ128ЃЎ4
I464ЃЎ577ЃЎ498ЃЎ9109ЃЎ2112ЃЎ0
I5392ЃЎ1113ЃЎ9138ЃЎ3141ЃЎ3153ЃЎ7
ЪдЛиД№ЃКБэжаПЩФмЮЊЗЧН№ЪєдЊЫиЕФЪЧ ЃЈЬюзжФИЃЉЃЛШєDЁЂEЮЊЭЌжмЦкЯрСкдЊЫиЃЌБэжаDБШEЕФЕквЛЕчРыФмТдДѓЃЌЦфдвђЪЧ ЁЁЁЁЁЁ ЁЃ