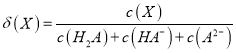��Ŀ����
����Ŀ��50 mL 0.50 mol/L������50 mL 0.55 mol/L NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________��
��2���ձ���������ֽ����������________________________��
��3�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ_____������ƫ������ƫС������Ӱ��������
��4��ʵ���и���60 mL 0.50 mol/L�����50 mL 0.55 mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������________�����������������������������к���________��������������������������������______________________________________��
���𰸡� ���β�������� ����ʵ������е�������ʧ ƫС ����� ��� �������������������Լ�������Ķ����йأ�������![]() ��Һ��
��Һ��![]() ��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����
��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����![]() ˮʱ�ų����ȣ��к������
ˮʱ�ų����ȣ��к������
����������1�����к��Ȳⶨ�м������ȼƹ����֪����ȱ�ٻ��β����������
��2����ֽ���������Ǽ���ʵ�������������ʧ��ʹʵ��ⶨ���к�������С��
��3�����ձ�����Ӳֽ�壬��ʵ������оͻ���������ʧ���Ӷ�ʹ�¶ȼƲ�õ��¶�ƫ�ͣ�ʹ�к�����ֵƫС��
��4���к�����ָ�������кͷ�Ӧ����1 mol H2O���ų��������������������ء�

 Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д�����Ŀ��ʳ�ö�������Ư����ʳƷ��������ĸΡ��������������ij����С�����ʵ�������������Ư���ԡ��ش��������⣺
��һ������������Ʊ�
ʵ����һ������������������ᣨŨ������ˮ1:1��ϣ���Ӧ��ȡ��������


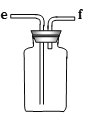
A B C D E
��1�����ռ�һƿ����Ķ�������ѡ����ͼ�е�װ�ã����������˳��Ϊ��________��������������Сд��ĸ��ʾ����
�����������������ʵļ���
�������ռ�����SO2ͨ������װ���У���һ���¶��°�ͼʾװ�ý���ʵ�顣
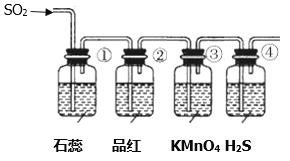
��2����������ʵ�飬�ش��������⣺
��� | ʵ������ | ����ԭ�� |
�� | _________________________ | _________________________ |
�� | Ʒ����Һ��ɫ | SO2����Ư���� |
�� | ________________________ | ��Ӧ�����ӷ���ʽ____________________ |
�� | ��Һ����ǣ��л�ɫ�������� | SO2+2H2S=3S��+2H2O |
��3��ʵ���з���Na2SO3���ܲ��ֱ��ʣ�����ⶨNa2SO3�Ĵ��ȣ���15.0g Na2SO3��Ʒ�����250mL��Һ��ȡ25.00mL��Һ����0.20 mol��L�D1����KMnO4��Һ���еζ����ﵽ�ζ��յ�ʱ����KMnO4��Һ20.00mL���ﵽ�ζ��յ�ʱ��ƿ����Һ��ɫ�仯��________________________����Ʒ��Na2SO3����������_______��