��Ŀ����
����Ŀ����Ҫ������
(1)FeCl3ˮ��Һ�����ԣ�ԭ����(�����ӷ���ʽ��ʾ)��___________________��ʵ��������FeCl3��Һ�ķ�����__________________________��
(2)��ĭ������װ��Al2(SO4)3��Һ��NaHCO3��Һ�����ݼ���д��ʹ��ʱ������Ӧ�����ӷ���ʽ____________________________
(3)д�����ܵ���ʵ��ܶȻ�����ʽ��Mg(OH)2��____________����Mg(OH)2����Һ�м���MgCl2���壬ƽ��________�ƶ�(�����������)��Ksp________(�������С�����䡱)��
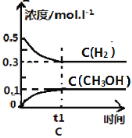
(4)1 g̿��ˮ������Ӧ����CO��H2��������a kJ�������˷�Ӧ���Ȼ�ѧ����ʽΪ______________________________________________��
(5)�������ʱ������(N2H4)Ϊȼ�ϣ��Զ������������������������Ӧ���ɵ�����ˮ��������֪��N2(g)��2O2(g)===2NO2(g)����H����67.7 kJ��mol��1��N2H4(g)��O2(g)===N2(g)��2H2O(g)����H����534 kJ��mol��1����N2H4��NO2��Ӧ���Ȼ�ѧ����ʽ___________��
���𰸡�Fe3����3H2O![]() Fe(OH)3��3H��������������ˮ��Al3����3HCO
Fe(OH)3��3H��������������ˮ��Al3����3HCO![]() ===Al(OH)3����3CO2��Ksp��[Mg2��][OH��]2����C(s)��H2O(g)===CO(g)��H2(g)��H����12a kJ��mol��12N2H4(g)��2NO2(g)===3N2(g)��4H2O(g)����H����1 135.7 kJ��mol��1
===Al(OH)3����3CO2��Ksp��[Mg2��][OH��]2����C(s)��H2O(g)===CO(g)��H2(g)��H����12a kJ��mol��12N2H4(g)��2NO2(g)===3N2(g)��4H2O(g)����H����1 135.7 kJ��mol��1
��������
��1��Fe3����ˮ�ⷴӦ����ʽΪFe3����3H2O![]() Fe(OH)3��3H����Ϊ��������ʱ����ˮ��������ǣ�Ӧ����������������ˮ�⣬�ʴ�Ϊ��Fe3����3H2O
Fe(OH)3��3H����Ϊ��������ʱ����ˮ��������ǣ�Ӧ����������������ˮ�⣬�ʴ�Ϊ��Fe3����3H2O![]() Fe(OH)3��3H����������������ˮ������2����ĭ����Ϊԭ��ΪAl3����3HCO
Fe(OH)3��3H����������������ˮ������2����ĭ����Ϊԭ��ΪAl3����3HCO![]() ===Al(OH)3����3CO2������3��Mg(OH)2�ܶȻ�����ʽΪKsp��[Mg2��][OH��]2����Mg(OH)2����Һ�м���MgCl2����ʱ��������Mg2��Ũ�ȣ��ܽ�ƽ�������ƶ��������ܶȻ��Dz���ģ��ʴ�Ϊ��Ksp��[Mg2��][OH��]2����������������4��1 g̿��ˮ������Ӧ����CO��H2��������a kJ��������1mol̿��Ӧ����12akJ�����������Ȼ�ѧ��Ӧ����ʽΪC(s)��H2O(g)===CO(g)��H2(g)��H����12a kJ��mol��1����5���ɸ�˹���ɿɵ�N2H4��NO2��Ӧ���Ȼ�ѧ����ʽΪ��2N2H4(g)��2NO2(g)===3N2(g)��4H2O(g)����H����1 135.7 kJ��mol��1��
===Al(OH)3����3CO2������3��Mg(OH)2�ܶȻ�����ʽΪKsp��[Mg2��][OH��]2����Mg(OH)2����Һ�м���MgCl2����ʱ��������Mg2��Ũ�ȣ��ܽ�ƽ�������ƶ��������ܶȻ��Dz���ģ��ʴ�Ϊ��Ksp��[Mg2��][OH��]2����������������4��1 g̿��ˮ������Ӧ����CO��H2��������a kJ��������1mol̿��Ӧ����12akJ�����������Ȼ�ѧ��Ӧ����ʽΪC(s)��H2O(g)===CO(g)��H2(g)��H����12a kJ��mol��1����5���ɸ�˹���ɿɵ�N2H4��NO2��Ӧ���Ȼ�ѧ����ʽΪ��2N2H4(g)��2NO2(g)===3N2(g)��4H2O(g)����H����1 135.7 kJ��mol��1��

����Ŀ�������ۺ�ͭ�۵ľ��Ȼ����ƽ���ֳ��ĵȷݣ��ֱ����ͬŨ�ȵ�ϡ���ᣬ��ַ�Ӧ���ڱ�״��������NO�������ʣ��������������±���������Ļ�ԭ����ֻ��NO�������м�������ȷ����
��� | �� | �� | �� | �� |
�������/mL | 100 | 200 | 300 | 400 |
ʣ�����/g | 18.0 | 9.6 | 0 | 0 |
NO���/L | 2.24 | 4.48 | 6.72 | V |
A. ������Һ����Fe3+ B. �����Ũ��Ϊ4mol/L
C. �����ܽ���9.6gCu D. ����V=6.72