��Ŀ����
 ��ҵ�����ð�����������һϵ�з�Ӧ�����Ʊ����ᣮ
��ҵ�����ð�����������һϵ�з�Ӧ�����Ʊ����ᣮ��1�������������Ļ�ѧ����ʽΪ
��2��ij�����ų���β����NOx�ĺ���Ϊ0.56%��������������ð������Խ���ת��Ϊ�����壬�����ķ�ӦΪ��6NOx+4xNH3=��3+2x��N2+6xH2O ������1��104L����״������β����42.5gNH3����x=
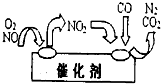
��3��NO��CO��������β���е��к����ʣ�������д�����Ϊ������Ⱦ������������װβ������װ�ã�����װ����װ�д����������ڴ�������������������õĻ�������ͼ��ʾ��д�����������е��ܻ�ѧ��Ӧ����ʽ��
��������1���������л�ԭ�ԣ��������������ԣ������е�Ԫ��Ϊ-3�ۣ�����������Ϊ+2�۵ĵ�Ԫ�أ���������������Ӧ����һ��������ˮ���ݴ˷������
��2�������NOx�������������n=
����NOx�����ʵ���������n=
���㰱�������ʵ������������ʵ���֮�ȵ��ڻ�ѧ������֮�ȼ���x��ֵ��
��3��NO2Ϊ�м�����Ӧ��ΪNO��O2��CO������ΪCO2��N2�����ݷ�Ӧ�������������غ㶨����ƽ����ʽ��
��2�������NOx�������������n=
| V |
| V m |
| m |
| M |
��3��NO2Ϊ�м�����Ӧ��ΪNO��O2��CO������ΪCO2��N2�����ݷ�Ӧ�������������غ㶨����ƽ����ʽ��
����⣺��1��NH3�����O2�ڴ��������������·�����Ӧ����һ��������ˮ����Ӧ����Ϊ��4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O��
��2��1��104Lβ����NOx�����Ϊ��1��104L��0.56%=56L����״����NOx�����ʵ���Ϊ
=2.5mol��42.5gNH3�����ʵ���Ϊ
=2.5mol�����ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ�����2.5mol��2.5mol=6��4x�����x=1.5��
�ʴ�Ϊ��1.5��
��3����ͼ��֪��NO2Ϊ�м�����Ӧ��ΪNO��O2��CO������ΪCO2��N2����Ӧ����ʽΪ2NO+O2+4CO=4CO2+N2��
�ʴ�Ϊ��2NO+O2+4CO
4CO2+N2��
| ||
| �� |
�ʴ�Ϊ��4NH3+5O2
| ||
| �� |
��2��1��104Lβ����NOx�����Ϊ��1��104L��0.56%=56L����״����NOx�����ʵ���Ϊ
| 56L |
| 22.4L/mol |
| 42.5g |
| 17g/mol |
�ʴ�Ϊ��1.5��
��3����ͼ��֪��NO2Ϊ�м�����Ӧ��ΪNO��O2��CO������ΪCO2��N2����Ӧ����ʽΪ2NO+O2+4CO=4CO2+N2��
�ʴ�Ϊ��2NO+O2+4CO
| ||
���������⿼��ѧ���������غ㶨�ɵ�������Ӧ�á���ѧʽ����ʽ����д�Լ����ݻ�ѧ����ʽ���м���Ľ�����������Ŀ�Ѷ��еȣ�ע�⣨3������ͼʾ�ҳ�NO2Ϊ�м�����ǽ��Ĺؼ���

��ϰ��ϵ�д�
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
�����Ŀ

