��Ŀ����
(17��)ʵ����������ƿʧȥ��ǩ���ᣬ�ֱ���Ũ���ᡢŨ�����Ũ���ᡣ
��1����ͬѧ��Ϊ���ý���ͭ���Լ��ɼ������������ᣬ���û�ѧ����ʽ�ͱ�Ҫ�����ּ���˵����____________________________________________________________________��
��2�������һ��ʵ��װ�ã�ʹͭ��ϡ���ᷴӦ��������ͭ��������������ķ����ڻ���װ��ͼ���������缫�������ƺ͵������Һ���ơ�

��3�� ʵ����������Ũ��������2.0mol��L-1��ϡ����500mL��
��ʵ���������������������Ͳ���ձ����������⣬����Ҫ��������____________________��
�����в��������������ҺŨ��ƫ�͵���_____________��
a������Ͳ��ȡŨ����ʱ���ӿ̶���
b������ʱ���ӿ̶���
c��ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ���
d�����ݺ�ҡ�ȣ�����Һ����ڿ̶ȣ�δ��������ˮ���̶�
��4����ͼ��ʾΪʵ����ģ�ҵ�����ð����������Ʊ������ʵ��

��װ��A�Ʊ����ﰱ����װ��B�Ʊ�����������
��װ��A���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________ ��
װ��B����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪ__________________________________��
�����й���A��Bװ�õ���������ȷ����__________
a����ѡ����ʵ��Լ�����Bװ��Ҳ���Ʊ�����
b��ʵ������У�A��Bװ����һ����һ������������ԭ��Ӧ
c��U�ι��е��Լ�������ͬ�������ò���ͬ
�۰���a��c��b��d��˳������װ�ý���ʵ�顣
�������ڷ�����Ӧ�Ļ�ѧ����ʽΪ___________________________________��
ʵ�������ijͬѧ���װ��C���Թ���������Һ��pH��7���ó��Ľ���Ϊ����Һһ�������ᡣ�ý���__________������ܡ������ܡ��������������_____________________________��
��1��Cu+2H2SO4=CuSO4+SO2��+2H2O
Cu+4HNO3=Cu(NO3)2+2NO2��+2H2O
��2��
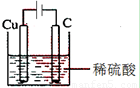
��3�� ��500mL����ƿ����ͷ�ιܣ���c
��4����2NH4Cl+Ca(OH)2=CaCl2+2NH3��+2H2O 2H2O2=H2O+O2��
��c��4NH3+5O2=4NO+6H2O�������ܣ���������ʱ���������ᷴӦ����NH4NO3��NH4NO3ˮ�������ԡ�
����������

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�


