��Ŀ����
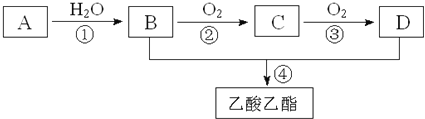
����Ŀ�����ɵ��������ܸ����Ŀ������ĸо�����Ҫ���㾫���ƾ���ˮ���ɵ���ˮ���ܰ�����ʿ���������㾫���溬���������ʣ���ҵ����AΪ��Ҫԭ�����ϳ�������������ϳ�·������ͼ��ʾ������A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����֪2CH3CHO+O2![]() 2CH3COOH����ش��������⣺
2CH3COOH����ش��������⣺
��1��д��A�ĵ���ʽ__________��
��2��B��D�����ں��еĹ����ŷֱ���__________��__________�������ƣ���
��3��д�����з�Ӧ�ķ�Ӧ���ͣ���______________����______________��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
��__________________________________________________��
��__________________________________________________��
��__________________________________________________��
���𰸡�![]() �ǻ��Ȼ��ӳɷ�Ӧȡ����Ӧ(����������Ӧ)CH2=CH2+H2O
�ǻ��Ȼ��ӳɷ�Ӧȡ����Ӧ(����������Ӧ)CH2=CH2+H2O![]() CH3CH2OH2CH3CH2OH+O2
CH3CH2OH2CH3CH2OH+O2![]() 2CH3CHO+2H2OCH3COOH+CH3CH2OH
2CH3CHO+2H2OCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O
��������
A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ��A����ϩ����ˮ�����ӳɷ�Ӧ����B���Ҵ���B��������������C����ȩ������2CH3CHO+O2![]() 2CH3COOH��֪D�����ᣬB��D����������Ӧ���������������ݴ˽��
2CH3COOH��֪D�����ᣬB��D����������Ӧ���������������ݴ˽��
�������Ϸ�����֪A����ϩ��B���Ҵ���C����ȩ��D�����ᣬ��
��1����ϩ�Ľṹ��ʽΪCH2��CH2������ʽΪ![]() ��
��
��2��B��D�ֱ����Ҵ������ᣬ�����ں��еĹ����ŷֱ����ǻ����Ȼ���
��3���������Ϸ�����֪���Ǽӳɷ�Ӧ������ȡ����Ӧ����������Ӧ��
��4��������ϩ��ˮ�ӳ������Ҵ�������ʽΪCH2��CH2+H2O![]() CH3CH2OH�������Ҵ��Ĵ�����������ʽΪ2CH3CH2OH+O2
CH3CH2OH�������Ҵ��Ĵ�����������ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O������������Ӧ������ʽΪCH3COOH+CH3CH2OH
2CH3CHO+2H2O������������Ӧ������ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��

����Ŀ��ij�о���ѧϰС����Ũ��Ϊ0.20mol��L-1�������Һ�ζ�����һ�������ʵ��ռ���Ʒ(���������Ӧ)����ˮ�γɵ���Һ��

��1��ȷ��ȡһ������Ĵ���Һ��Ҫʹ�õ�������______________��
��2�����ζ���ʼ�ͽ���ʱ���ζ����е�Һ����ͼ��ʾ�������������Һ�����Ϊ________mL��
��3���ζ�ʱ�����Է�̪Ϊָʾ�����ζ��ﵽ�յ�ı�־��______________________________��
��4����ȷ��ȡ��5.0g�ռ���Ʒ���Ƴ�250mL����Һ�����������Һ�ζ����ζ�ǰ�������ζ���������±���ʾ��
�ζ����� | ����Һ���(mL) | 0.20mol��L-1���������(mL) | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 10.00 | 0.70 | 20. 60 |
��һ�� | 10.00 | 4.00 | 24.10 |
������ | 10.00 | 1.10 | 21.10 |
��ʵ�����ݿ�֪���ռ�Ĵ���Ϊ__________��
��5�����в����ᵼ�²�õĴ���Һ��Ũ��ƫ�����________(����ĸ)��
a.���ֱ�Һ�γ���ƿ�� b.�ô�����Һ��ϴ��ƿ
C.��ƿϴ������������ˮ d.�ų���Һ�ĵζ��ܿ�ʼ�����ݣ��ų�Һ���������ʧ



