МвДҝДЪИЭ
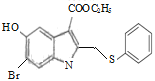
ЎҫМвДҝЎҝ°ўұИ¶д¶ыЦчТӘУГУЪјЧЎўТТРНБчёРәНЖдЛьәфОьөАІЎ¶ҫёРИҫЦўөД·АЦОЈ¬УРЧЁјТИПОӘЖдФЪРВ№Ъ·ОСЧөДЦОБЖЙПТІУРТ»¶ЁөДБЖР§ЎЈ°ўұИ¶д¶ыөДәПіЙВ·ПЯИзНјЛщКҫЈә

(1)НјЦРIОӘ°ўұИ¶д¶ыЈ¬Жд·ЦЧУКҪОӘ__________Ј¬
(2) AөДГыіЖКЗ__________Ј¬GЎъHөД·ҙУҰАаРНОӘ___________ЎЈ
(3) CЦРЛщә¬өДә¬Сх№ЩДЬНЕөДГыіЖОӘ_____________ЎЈ
(4) FЎъGөД»ҜС§·ҪіМКҪОӘ____________ЎЈ
(5)CУР¶аЦЦН¬·ЦТм№№МеЈ¬РҙіцДЬН¬КұВъЧгПВБРМхјюөД·јПг»ҜәПОпөДҪб№№јтКҪ(І»ҝјВЗБўМеТм№№)_________ЎЈ
ўЩДЬУлNaHCO3ИЬТә·ҙУҰ·ЕіцCO2Ј»ўЪәЛҙЕ№ІХсЗвЖЧУРИэЧй·еЈ¬ЗТ·еГж»эөДұИКЗ1:1:1ЎЈ
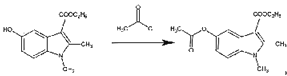
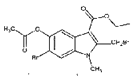
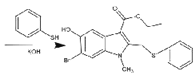
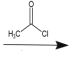
(6)ИзНјОӘәПіЙ°ўұИ¶д¶ыөДБнТ»ЦЦ·Ҫ·ЁЈ¬ЗлІОХХЙПКцБчіМҪ«ПВБРБчіМНјІ№ідНкХы(КФјБҝЙҙУЙПМвКФјБЦРИОСЎ)Јә______

Ўҫҙр°ёЎҝC22H25BrN2O3S 3-өвұҪ·У ИЎҙъ·ҙУҰ Пх»щ хҘ»щ 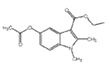 +2Br2Ўъ
+2Br2Ўъ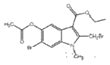 +2HBr
+2HBr 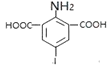 Ўў
Ўў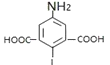
![]()
![]()

ЎҫҪвОцЎҝ
ЈЁ1Ј©ёщҫЭ°ўұИ¶д¶ыөДҪб№№јтКҪЈ¬НЖІвЖд·ЦЧУКҪЈ»
ЈЁ2Ј©·јПгЧе»ҜәПОпөДГьГыЈ¬КЧПИИ·¶ЁДёМеЈ¬И»әу¶ЁИЎҙъ»щөДО»ЦГЈ»ТФј°УР»ъ»ҜәПОп·ҙУҰөДМШөгЈ¬ҪбәПGЎўHөДҪб№№јтКҪЈ¬И·¶ЁGЎъHөД·ҙУҰАаРНЈ»
ЈЁ3Ј©ёщҫЭCөДҪб№№јтКҪЈ¬ХТіцC·ЦЧУЦРЛщә¬өД№ЩДЬНЕЈ»
ЈЁ4Ј©ҪбәПFЎўGөДҪб№№јтКҪЈ¬И·¶ЁFУлBr2·ўЙъөДКЗИЎҙъ·ҙУҰЈ¬ТФҙЛКйРҙ·ҪіМКҪЈ»
ЈЁ5Ј©ёщҫЭCөДН¬·ЦТм№№МеРиТӘВъЧгөДМхјюЈ¬ХТіц·ыәПМхјюөДCөДН¬·ЦТм№№МеЈ»
ЈЁ6Ј©ёщҫЭ°ўұИ¶д¶ыөДәПіЙВ·ПЯНјЦРЛщЙжј°өД·ҙУҰОпЎўЙъіЙОпЎў·ҙУҰМхјюЈ¬әПАнАыУГХвР©РЕПўИҘ·ЦОцЈ¬ИҘЙијЖәПіЙ°ўұИ¶д¶ыөДБнТ»ЦЦ·Ҫ·ЁЎЈ
ЈЁ1Ј©УЙ°ўұИ¶д¶ыөДҪб№№јтКҪҝЙЦӘЈ¬°ўұИ¶д¶ыөД·ЦЧУКҪОӘC22H25BrN2O3SЈ»
ЈЁ2Ј©IФӯЧУУл·УфЗ»щФЪұҪ»·ЙПО»УЪјдО»өД№ШПөЈ¬ЖдГыіЖОӘ3-өвұҪ·УЈ»GУл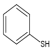 ФЪKOHЧчУГПВЈ¬·ўЙъИЎҙъ·ҙУҰЙъіЙHЈ»
ФЪKOHЧчУГПВЈ¬·ўЙъИЎҙъ·ҙУҰЙъіЙHЈ»
ЈЁ3Ј©CЦРЛщә¬өДә¬№ЩДЬНЕөДГыіЖОӘхҘ»щЎўПх»щЎўөвФӯЧУЈ¬№КCЦРЛщә¬өДә¬Сх№ЩДЬНЕөДГыіЖОӘхҘ»щЎўПх»щЈ»
ЈЁ4Ј©FУлBr2·ўЙъИЎҙъ·ҙУҰЙъіЙGәНHBrЈ¬Жд·ҙУҰ·ҪіМКҪОӘ 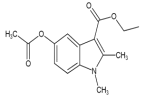 +2Br2Ўъ
+2Br2Ўъ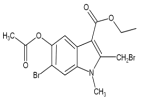 +2HBrЈ»
+2HBrЈ»
ЈЁ5Ј©CУР¶аЦЦН¬·ЦТм№№МеЈ¬ўЩДЬУлNaHCO3ИЬТә·ҙУҰ·ЕіцCO2Ј¬ЛөГчёГОпЦКЦРә¬УР-COOHЈ»ўЪәЛҙЕ№ІХсЗвЖЧУРИэЧй·еЈ¬ЗТ·еГж»эөДұИКЗ1:1:1Ј¬№КёГОпЦКұҪ»·ЙПә¬УР2ёц-COOHЈ¬1ёц-NH2Ј¬1ёц-IЈ¬№К·ыәПМхјюөДCөДН¬·ЦТм№№МеОӘ Ўў
Ўў Ј»
Ј»
ЈЁ6Ј©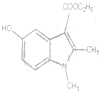 Ул
Ул![]() ·ўЙъИЎҙъ·ҙУҰЙъіЙ
·ўЙъИЎҙъ·ҙУҰЙъіЙ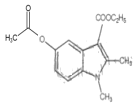 Ј¬
Ј¬ УлBr2·ўЙъИЎҙъ·ҙУҰЙъіЙ
УлBr2·ўЙъИЎҙъ·ҙУҰЙъіЙ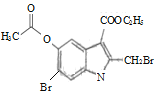 Ј¬
Ј¬ Ул
Ул ·ўЙъИЎҙъ·ҙУҰЙъіЙ
·ўЙъИЎҙъ·ҙУҰЙъіЙ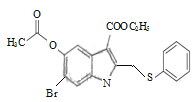 Ј¬әПіЙВ·ПЯИзПВЈә
Ј¬әПіЙВ·ПЯИзПВЈә

![]()


![]()
 ЎЈ
ЎЈ

ЎҫМвДҝЎҝўс.»рјэНЖҪшЖчЦРКўУРЗҝ»№ФӯјБТәМ¬лВ(N2H4)әНЗҝСх»ҜјБТәМ¬Л«СхЛ®ЎЈөұЛьГЗ»мәП·ҙУҰКұЈ¬јҙІъЙъҙуБҝөӘЖшәНЛ®ХфЖшЈ¬Іў·ЕіцҙуБҝИИЎЈТСЦӘЈә0.4 molТәМ¬лВУлЧгБҝөДТәМ¬Л«СхЛ®·ҙУҰЈ¬ЙъіЙөӘЖшәНЛ®ХфЖшЈ¬·Еіц256 kJөДИИБҝЎЈ
(1)ёГ·ҙУҰөДИИ»ҜС§·ҪіМКҪОӘ_______________________________________ЎЈ
(2)УЦЦӘH2O(l)=H2O(g)ЎЎҰӨHЈҪЈ«44 kJЎӨmolЈӯ1Ј¬Фт16 gТәМ¬лВУлТәМ¬Л«СхЛ®·ҙУҰЙъіЙТәМ¬Л®Кұ·ЕіцөДИИБҝКЗ________kJЎЈ
(3)ҙЛ·ҙУҰУГУЪ»рјэНЖҪшЈ¬іэКН·ЕҙуБҝИИәНҝмЛЩІъЙъҙуБҝЖшМеНв»№УРТ»ёцәЬҙуөДУЕөгКЗ________________________ЎЈ
ўтЈ®ІОҝјПВБРНјұнәНУР№ШТӘЗу»ШҙрОКМвЎЈ
(1)ИзНјКЗ1 mol NO2(g)әН1 mol CO(g)·ҙУҰЙъіЙCO2(g)әНNO(g)№эіМЦРДЬБҝұд»ҜКҫТвНјЈ¬ЗлРҙіцNO2әНCO·ҙУҰөДИИ»ҜС§·ҪіМКҪЈә__________________________________Ј»
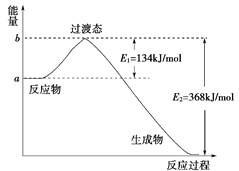
(2)ПВұнКЗІҝ·Ц»ҜС§јьөДјьДЬКэҫЭЈә
»ҜС§јь | PЎӘP | PЎӘO | O=O | P=O |
јьДЬ/(kJ/mol) | a | b | c | x |
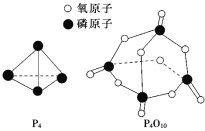
ТСЦӘ°ЧБЧөДИјЙХИИҰӨHЈҪЈӯd kJ/molЈ¬°ЧБЧј°ЖдНкИ«ИјЙХөДІъОпҪб№№ИзНјЛщКҫЈ¬ФтЙПұнЦРxЈҪ______________________(УГә¬УРaЎўbЎўcЎўdөДҙъКэКҪұнКҫ)ЎЈ