��Ŀ����
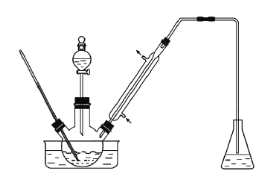
����Ŀ��ʵ�����Ա���ȩΪԭ���Ʊ����屽��ȩ��ʵ��װ�ü���ͼ��������ʵķе����������
���� | �е�/�� | ���� | �е�/�� |
�� | 58��8 | 1��2-�������� | 83��5 |
����ȩ | 179 | ���屽��ȩ | 229 |
��ʵ�鲽��Ϊ��
����1��������ƿ�е�һ����ȵ���ˮAlCl3��1��2����������ͱ���ȩ��ֻ�Ϻ�������60�棬�����μӾ�Ũ����������Һ�壬���·�Ӧһ��ʱ�䣬��ȴ��
����2������Ӧ����ﻺ������һ������ϡ�����У����衢���á���Һ���л�����10%NaHCO3��Һϴ�ӡ�
����3����ϴ�ӵ��л������������ˮMgSO4���壬����һ��ʱ�����ˡ�
����4����ѹ�����л��࣬�ռ���Ӧ��֡�
(1) ʵ��װ���������ܵ���Ҫ������___________________��
(2) ����1������������У���һ�������Ǵ������仯ѧʽΪ__________________
(3) д���ɱ���ȩ��ȡ���屽��ȩ�Ļ�ѧ����ʽ_____________________________________________________
(4) Ϊ��ֹ��Ⱦ��������ƿ��ʢװ����ҺӦ����______________________________
���𰸡�������������߷�Ӧ���ת���� AlCl3  NaOH��Һ
NaOH��Һ
��������
�����Լ��屽��ȩ�ĺϳ�Ϊ���壬�ۺϿ������������á���ѧ����ʽ����д��ʵ����Ⱦ�ķ��ε����е��Ѷȡ�
(1)����װ�õ�������Ӧ��������������ƿ�еĻӷ������ʡ����е��塢1,2-��������е�ϵͣ��������ǵ���������߷�Ӧ���ת���ʡ�
(2)����ƿ��������������У�����ȩ��Һ���Ǻϳɼ��屽��ȩ��ԭ�ϣ�1��2�����������Ƿ�Ӧ�ܼ�������ˮAlCl3Ϊ��Ӧ�Ĵ�����
(3) ����ȩ��ȡ���屽��ȩ�Ļ�ѧ����ʽ ��
��
(4) �ϳɷ�Ӧ���ɵ�HBrΪ�ж����壬Ϊ��ֹ����Ⱦ��������ƿ��ӦʢװNaOH��Һ��

����Ŀ����������ʵ��������������ó��Ľ�����ȷ����
ѡ�� | ʵ����������� | ���� |
A | �������м���Ũ���ᣬ����ú�ɫ����״���壬���ų��̼�����ζ���� | Ũ�����������ˮ�Ժ���ˮ�� |
B | ����ɫʯ����ֽ�ϵμ�������ˮ����ֽ��Ե�ʺ�ɫ���м�Ϊ��ɫ | ��ˮ�����������л�ԭ�� |
C | ±����Y��NaOH��Һ���Ⱥ�������ϡ���ᣬ�ٵμ�AgNO3��Һ��������ɫ���� | ±����Y�к�����ԭ�� |
D | ȡ5 mL 0.1 mol��L��1KI��Һ������1 mL 0.1 mol��L��1FeCl3��Һ����ȡ��Һ����ˮ�����KSCN��Һ����Һ���Ѫ��ɫ | Fe3����I���������ķ�ӦΪ���淴Ӧ |
A. A B. B C. C D. D

