��Ŀ����
15����ѧʽΪC3H8O�Ļ�����A��������������A+Na��������������
��A+CH3COOH$��_{��}^{ŨH_{2}SO_{4}}$����ζ������ C
��A�������IJ���B�ܷ���������Ӧ
��1��д���ṹ��ʽACH3CH2CH2OH BCH3CH2CHO
��2��A��Ũ���������·�����ȥ��Ӧ���ɵ��л�����Ľṹ��ʽCH3CH=CH2
��3��A��CH3COOH��Ӧ����C�ķ�Ӧ����������ȡ������Ӧ��
���� ��A+Na�������������ݣ�˵��A�к���-OH��
��A+CH3COOH$��_{��}^{ŨH_{2}SO_{4}}$����ζ������C��˵��A�����ᷢ��������Ӧ����Ϸ���ʽ��֪AӦΪ��������ΪCH 3CH2CH2OH ��CH3CH��OH��CH3��
��A�������IJ���B�ܷ���������Ӧ��˵����B����ȩ������AΪCH 3CH2CH2OH������BΪCH3CH2CHO��CΪCH3COOCH2CH2CH3����϶�Ӧ�л���Ľṹ�����ʽ�����
��� ���A+Na�������������ݣ�˵��A�к���-OH��
��A+CH3COOH$��_{��}^{ŨH_{2}SO_{4}}$����ζ������C��˵��A�����ᷢ��������Ӧ����Ϸ���ʽ��֪AӦΪ��������ΪCH 3CH2CH2OH ��CH3CH��OH��CH3��
��A�������IJ���B�ܷ���������Ӧ��˵����B����ȩ������AΪCH 3CH2CH2OH������BΪCH3CH2CHO��CΪCH3COOCH2CH2CH3��
��1�������Ϸ�����֪AΪCH 3CH2CH2OH��BΪCH3CH2CHO��
�ʴ�Ϊ��CH3CH2CH2OH ��CH3CH2CHO��
��2��AΪCH 3CH2CH2OH����Ũ���������·�����ȥ��Ӧ���ɵ��л����ﶼ��CH3CH=CH2��
�ʴ�Ϊ��CH3CH=CH2��
��3��AΪCH 3CH2CH2OH���������ᷢ��������ȡ������Ӧ��
�ʴ�Ϊ��������ȡ������Ӧ��
���� ���⿼���л�����ƶϣ��������л�������ŵ����ʵĿ��飬�ۺϿ���ѧ�����������ͻ�ѧ֪ʶ���ۺ�������������Ŀ�Ѷȴ�

 �����û�����ʱ��Ӧ�϶����������ϵ�̼ԭ����ĿΪ��������
�����û�����ʱ��Ӧ�϶����������ϵ�̼ԭ����ĿΪ��������| A�� | 9 | B�� | 10 | C�� | 11 | D�� | 12 |
| A�� | �ü�ʽ�ζ���ʢװ���������Һ | B�� | �������������ռ� | ||
| C�� | ����������ȥ������еĽᾧˮ | D�� | ����������ʱ����ʯ���� |
| A�� | 3�ֺ�4�� | B�� | 4�� ��1�� | C�� | 5�� ��2�� | D�� | 6�ֺ�4�� |
| A�� | ���ʵ���Ũ����ͬ����Һ���������ʵ�������ͬ | |
| B�� | ���³�ѹ�£�0.1molH2����Լ��6.02��1022��H2���� | |
| C�� | 1mol•L-1������Һ100mL��H+�ĸ���ԼΪ0.1NA | |
| D�� | ��״���£�1molH2O�����Լ22.4L |
| A�� | ����£�11.2L SO3�����ķ�����ĿΪ0.5 NA | |
| B�� | 1L0.1 mol•L-1��̼��������Һ�к�̼�����������Ϊ0.1NA | |
| C�� | ���³�ѹ�£�1.7gH2O2�к��еĵ�����Ϊ0.9nA | |
| D�� | 8 Al+3 NH4ClO4�T4 Al2O3+3NH3+3HCl ��Ӧ�У�����27gAl��ʱ��ת�Ƶĵ�����ĿΪ2.75NA |
| A�� | Na+�Ļ�̬�����Ų�ͼ�ǣ� | |
| B�� | 7.8 gNa2S��7.8 gNa2O2�к��е���������Ŀ��Ϊ0.1NA | |
| C�� | Na2S�ĵ���ʽ�� | |
| D�� | �����ӽṹʾ��ͼΪ��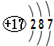 |
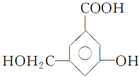
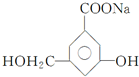 ��
��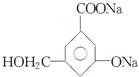 ��
�� ��
��