��Ŀ����
����Ŀ��ƻ���ף�ACV����һ����ƻ�����Ͷ��ɵ�������Ʒ�����нⶾ����֬��ҩЧ����Ҫ��������Ϊƻ���ᡣƻ�����ڷ����ᴿ��Ļ�ѧ�������£�����Է�������������150����ȫȼ�պ�ֻ����CO2��H2O��������C��H���������ֱ�Ϊw��C����35��82����w��H����4��48������1 mol������������NaHCO3��Ӧ�ų�44��8 L CO2����������Na��Ӧ�ų�33��6 L H2�����������������Ϊ��״�������ۺ˴Ź���������ʾ��������ҷ����֮��Ϊ3:2:1��
ͨ������ش��������⣺
��1��ƻ�����к��еĹ���������Ϊ____________________��
��2��ƻ����ķ���ʽ________________��
��3��д��ƻ����Ľṹ��ʽ _____________________��
��4��ƻ�����ͬ���칹���У����������ڵ���__________��
��5��ƻ���������ᷢ����Ӧ�ķ���ʽ______________________��
���𰸡� �ǻ����Ȼ� C4H6O5 


![]() +HCOOH
+HCOOH![]()
 +H2O
+H2O
��������ƻ���������w(C)=35.82%��w(H)=4.48%����O����������Ϊ1-35.82%-4.48%=59.7%����Է�������������150����Oԭ�������Ŀ=![]() =5.6��1molƻ������������NaHCO3��Ӧ�ų�44.8L CO2��������̼���ʵ���Ϊ2mol��˵�������к���2��-COOH����������Na��Ӧ�ų�33.6L H2����������Ϊ1.5mol��2mol-COOH��������1mol�������ʷ����л�����1��-OH����������ƻ������Ӻ���5��Oԭ�ӣ���ƻ�������Է�������=
=5.6��1molƻ������������NaHCO3��Ӧ�ų�44.8L CO2��������̼���ʵ���Ϊ2mol��˵�������к���2��-COOH����������Na��Ӧ�ų�33.6L H2����������Ϊ1.5mol��2mol-COOH��������1mol�������ʷ����л�����1��-OH����������ƻ������Ӻ���5��Oԭ�ӣ���ƻ�������Է�������=![]() =134���������Cԭ����Ŀ=
=134���������Cԭ����Ŀ=![]() =4��Hԭ����Ŀ=
=4��Hԭ����Ŀ=![]() =6�������ʽΪC4H6O5��
=6�������ʽΪC4H6O5��
(1)�ɷ�����֪��ƻ��������ں����Ȼ����ǻ���
(2)ƻ����ķ���ʽC4H6O5��
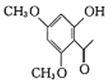
(3)�ɷ�����֪��ƻ�����������2��-COOH��1��-OH���˴Ź���������ʾ��������ҷ����֮��Ϊ3:2:1����֪ƻ������ӽṹΪ ��
��
(4)ƻ�����ͬ���칹���У����������ڣ�������ͬ��Ŀ�Ĺ����ţ�����ṹΪ
 ��
��
(5)ƻ����������(HCOOH)����������Ӧ�ķ���ʽΪ![]() +HCOOH
+HCOOH![]()
 +H2O��
+H2O��

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�����Ŀ���ڶ����뵼����ϡ�������-V)A�廯�������������ʹ֮��Ϊ��ѧ�ҵ��о��ȵ�֮һ��
(1) ��̬��ԭ�ӵļ۵��ӹ����ʾʽΪ_____________��
(2) N��P��Asλ��ͬһ���壬��̬��ԭ�ӵĺ����________�ֲ�ͬ�˶�״̬�ĵ��ӣ�N2O�Ŀռ乹��Ϊ_________��NH4NO3��N���ӻ���ʽΪ_________________����PO43-��Ϊ�ȵ�����ķ�����________________����һ�ּ��ɣ���
��3����֪NH3���ӵļ���ԼΪ107�㣬��PH3���ӵļ���ԼΪ94��,���ü۲���ӶԻ������۽���NH3�ļ��DZ�PH���ļ��Ǵ��ԭ��__________________________��
��4��������������Ԫ���У�����һ�����ܴ�С����һ������������֮���Ԫ����________________��
��5�������𡢵������������صĽṹ�����ڽ��ʯ���۵��������ʾ��
���� | BN | AIN | GaN |
�۵�/�� | 3000 | 2200 | 1700 |
�Դӽṹ�ĽǶȷ��������۵㲻ͬ��ԭ��_____________________��
��6������������ͼ��ʾ��A1ԭ�ӵ���λ��Ϊ________����������ԭ��֮����������Ϊd pm��NA���������ӵ�������ֵ�� ������������ܶȦ�=_________g/cm3��

����Ŀ����ʽ������(NiOOH)�����������ص��������ϣ����÷�����������Ҫ��Ni��A1������Cr��FeS��)���Ʊ����乤���������£�

�ش��������⣺
��1�������ݳ�����ʱ��������Ӧ�Ļ�ѧ����ʽΪ________________��
��2�����ܽ⡱ʱ�ų�������Ϊ_________���ѧʽ)��
��3��������1��ʱ�����������£���Һ�е�Fe2+������ΪFe3+�������ӷ���ʽΪ______________��
��4����֪�������½������ӿ�ʼ��������ȫ������pH���±���
��ʼ������pH | ��ȫ������pH | |
Ni2+ | 6.2 | 8.6 |
Fe2+ | 7.6 | 9.1 |
Fe3+ | 2.3 | 3.3 |
Cr3+ | 4.5 | 5.6 |
����pH 1��ʱ����ҺpH��ΧΪ__________������2���������ijɷ�Ϊ___________(�ѧʽ)��
��5��д���ڿ����м���Ni(OH)2��ȡNiOOH�Ļ�ѧ����ʽ______________��
��6�������Ȳ���֣����Ƶõ�NiOOH�л����Ni(OH)2������ɿɱ�ʾΪxNiOOH��y Ni(OH)2���ֳ�ȡ8.29g xNiOOH��y Ni(OH)2��Ʒ����ϡ���ᣬ��������Һ���壬������200mL,������ȡ20.00 mL,��0.010mol��L-1��KMnO4����Һ�ζ����ظ���������2�Σ�ƽ������KMnO4 ����Һ 20.00 mL����֪ 5Ni2++MnO4-+8H+=5Ni3++Mn2++4H2O����x=_________��y=_________��