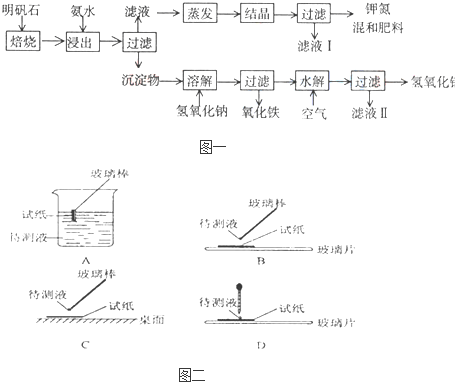
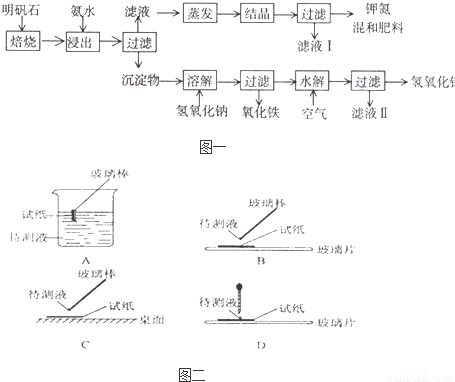
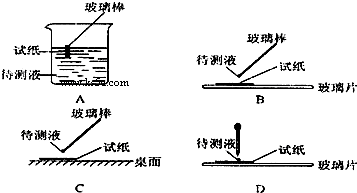
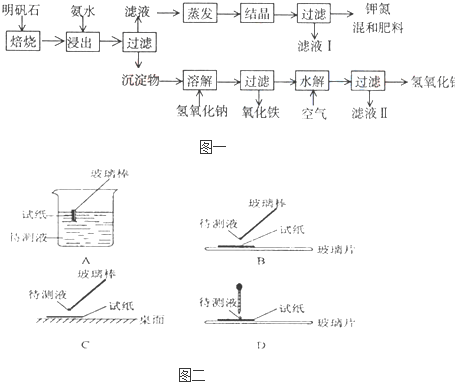
��Ŀ����
ij��ɫ����Һ���ܺ����������ӣ�K+��Al3+��Fe3+��Ba2+��NO3-��SO42-��HCO3-��Cl-��ȡ����Һ��������ʵ�飺
������ɫʯ����ֽ������Һ����ֽ�Ժ�ɫ��
��ȡ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬������������������Ϊ����ɫ��
��ȡ��Һ���������백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ��
��ȡ��Һ�����������Ȼ�����Һ������ɫ������
��ȡʵ�� �ܺ�ij�����Һ��������������Һ������ɫ�������ټ��������ϡ���ᣬ��������ʧ��
��ش��������⣺
��1����ʵ�� ���У���ͼ��ʾ�IJ�������ȷ����
��2����������ʵ���ж�ԭ��Һ�п϶����ڵ�������
��3��д����ڢ�����ʵ���йص����ӷ���ʽ��
��
��
������ɫʯ����ֽ������Һ����ֽ�Ժ�ɫ��
��ȡ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬������������������Ϊ����ɫ��
��ȡ��Һ���������백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ��
��ȡ��Һ�����������Ȼ�����Һ������ɫ������
��ȡʵ�� �ܺ�ij�����Һ��������������Һ������ɫ�������ټ��������ϡ���ᣬ��������ʧ��
��ش��������⣺
��1����ʵ�� ���У���ͼ��ʾ�IJ�������ȷ����
B
B
������ţ���
��2����������ʵ���ж�ԭ��Һ�п϶����ڵ�������
Al3+��NO3-��SO42-
Al3+��NO3-��SO42-
���϶������ڵ�������Fe3+��Ba2+��HCO3-
Fe3+��Ba2+��HCO3-
����3��д����ڢ�����ʵ���йص����ӷ���ʽ��
��
3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O
3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O
��
Al3++3NH3?H2O�TAl��OH��3��+3NH4+
Al3++3NH3?H2O�TAl��OH��3��+3NH4+
����������1����������ֽ������Һ�������ʱ��Ӧ�ò�����պȡ������Һ���ڷ��ڲ���Ƭ����ֽ�ϼ��������
��2�����ݷ�Ӧ�������ж����Ӵ��ڵĿ����ԣ�
��3���ڸ��ݷ�ӦΪ����ͭ����������������������ӷ���������ԭ��Ӧ����ͭ���Ӻ�һ������������д���ӷ���ʽ��
�۸���Al��OH��3������������ں�Al3+��Һ�м��백ˮֻ����Al��OH��3��д���ӷ���ʽ��
��2�����ݷ�Ӧ�������ж����Ӵ��ڵĿ����ԣ�
��3���ڸ��ݷ�ӦΪ����ͭ����������������������ӷ���������ԭ��Ӧ����ͭ���Ӻ�һ������������д���ӷ���ʽ��
�۸���Al��OH��3������������ں�Al3+��Һ�м��백ˮֻ����Al��OH��3��д���ӷ���ʽ��
����⣺��1������ֽ������Һ�������ʱ��Ӧ�ò�����պȡ������Һ���ڷ��ڲ���Ƭ����ֽ�ϼ��飬�ʴ�Ϊ��B��
��2��������ɫʯ����ֽ������Һ����ֽ�Ժ�ɫ��˵����Һ�����ԣ���һ��������HCO3-����Һ��ɫ������Fe3+��
��ȡ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬������������������Ϊ����ɫ��������ΪNO��˵����Һ�д���NO3-����
��ȡ��Һ���������백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ��˵������Al3+���ӣ���
��ȡ��Һ�����������Ȼ�����Һ������ɫ�������ó���Ϊ���ᱵ������˵������SO42-����һ�����Ậ��Ba2+��
��ȡʵ�� �ܺ�ij�����Һ��������������Һ������ɫ�������ټ��������ϡ���ᣬ��������ʧ������֤���Ƿ���Cl-���ӣ�����м����Ȼ�����
�ʴ�Ϊ��Al3+��NO3-��SO42-��Fe3+��Ba2+��HCO3-��
��3���ڷ�ӦΪ����ͭ����������������������ӷ���������ԭ��Ӧ����ͭ���Ӻ�һ���������壬��Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
�۸���Al��OH��3������������ں�Al3+��Һ�м��백ˮֻ����Al��OH��3����Ӧ�����ӷ���ʽΪAl3++3NH3?H2O�TAl��OH��3��+3NH4+��
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3��+3NH4+��
��2��������ɫʯ����ֽ������Һ����ֽ�Ժ�ɫ��˵����Һ�����ԣ���һ��������HCO3-����Һ��ɫ������Fe3+��
��ȡ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬������������������Ϊ����ɫ��������ΪNO��˵����Һ�д���NO3-����
��ȡ��Һ���������백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ��˵������Al3+���ӣ���
��ȡ��Һ�����������Ȼ�����Һ������ɫ�������ó���Ϊ���ᱵ������˵������SO42-����һ�����Ậ��Ba2+��
��ȡʵ�� �ܺ�ij�����Һ��������������Һ������ɫ�������ټ��������ϡ���ᣬ��������ʧ������֤���Ƿ���Cl-���ӣ�����м����Ȼ�����
�ʴ�Ϊ��Al3+��NO3-��SO42-��Fe3+��Ba2+��HCO3-��
��3���ڷ�ӦΪ����ͭ����������������������ӷ���������ԭ��Ӧ����ͭ���Ӻ�һ���������壬��Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
�۸���Al��OH��3������������ں�Al3+��Һ�м��백ˮֻ����Al��OH��3����Ӧ�����ӷ���ʽΪAl3++3NH3?H2O�TAl��OH��3��+3NH4+��
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3��+3NH4+��
���������⿼�����ӵļ��飬��Ŀ�ѶȲ�����ע�ⳣ�����Ӽ���ķ����Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
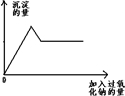 ij��ɫ����Һ�п��ܺ�Mg2+��Al3+��Fe3+��Cu2+��NH4+��K+�еļ������ӣ�����������Ĺ�������ʱ������ɫ��ζ�����������ͬʱ���ɰ�ɫ����������Ĺ������Ƶ����������������֮��Ĺ�ϵ����ͼ��ʾ���Իش�
ij��ɫ����Һ�п��ܺ�Mg2+��Al3+��Fe3+��Cu2+��NH4+��K+�еļ������ӣ�����������Ĺ�������ʱ������ɫ��ζ�����������ͬʱ���ɰ�ɫ����������Ĺ������Ƶ����������������֮��Ĺ�ϵ����ͼ��ʾ���Իش�