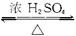��Ŀ����
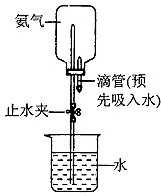
��ͼ��ʾ���Ӻ�װ��������
��֪��A��װ��NaNO2��NH4Cl�����Һ��B��װ�м�ʯ�ң�C��������Ͻ�D���з�̪��Һ��C�з�����Ӧ���Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)![]() 2NH3(g)����H=-92.4 kJ��mol-1��
2NH3(g)����H=-92.4 kJ��mol-1��
ʵ�鲽�����£��ټ��װ�õ������ԣ����þƾ��Ƽ���NH4Cl��NaNO2������Һ�������������ɣ���ֹͣ���ȣ������д�����������������þƾ��Ƹ�����Ͻ������һ���¶ȣ��ܴӽ�ͷ�ι��еμ�һ��Ũ�����ᣬ���ü���̪��Һ��졣
�ش��������⣺
(1)д��A������Һ�䷢����Ӧ�Ļ�ѧ����ʽ____________________________________���˷�ӦΪ________�ȷ�Ӧ(��š�������)��
(2)���þƾ��Ƽ���NH4Cl��NaNO2������Һ��Ŀ����__________________��
(3)C������Ͻ��������____________________________��Ҫ�ȼ�����һ���¶ȵ�ԭ����____________________________��
(4)���ͨ��C�е�N2��H2����1 mol��3 mol��Ϊʲô��Ӧ��ų���������92.4 kJ�٣�
(1)NaNO2+NH4Cl![]() N2+NaCl+2H2O����
N2+NaCl+2H2O����
(2)ʹNH4Cl��NaNO2������Һ������Ӧ��������
(3)�����������Ļ��Ը�
(4)��Ϊ�÷�Ӧ�ǿ��淴Ӧ��1 mol N2��3 mol H2��������ȫ��Ӧ�������Էų���������92.4 kJ�١�
������(1)����Ŀ��ʵ��Ŀ�Ŀ�֪���ϳɰ���һ��Ҫ�е��������÷�Ӧ������Ӧ���е�Ԫ�ؿɷ������з�Ӧ���ɵ��������ڸ÷�Ӧ����������ʱֹͣ���ȣ��������д���������������ƶ��Ƿ��ȷ�Ӧ��(3)�ϳɰ��ķ�Ӧ��������ɷ�Ӧ��Ϣ��֪���ô���ΪC�е�����Ͻ�(4)��Ϊ���淴Ӧ�и��������ʵ����Dz���������ģ��ʷų�������һ����92.4 kJ�١�

| ʵ����� | ʵ������ |
| ��Һ�ȱ�����ɫ | |
| �а�ɫ�������� | |
| �����ݲ�������ˮ��ɫ��dz |
��1����ʵ�������������з����Ʊ����������в��������ǣ�����ţ�
A������Ũ��ˮ
B������ʯ�Һ��Ȼ�淋Ļ�������
C����Ũ��ˮ�ε���ʯ����
D�������Ȼ�粒���
��2������������ȷ�ķ�����ѡ��ͼ��ʾ��װ����ȡ������д���Թ�����������Ӧ�Ļ�ѧ����ʽ
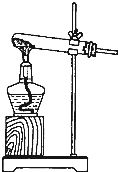
��3��ͬѧ����Ƶ��ռ������հ����ļ���װ������ͼ��ʾ�������������ռ��������ǣ�����ţ�
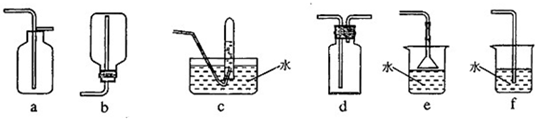
��4�������ռ��������ļ���ƿ����ͼ��ʾ���Ӻ�װ�ã��г�װ�ü���ȥ��������Ȫʵ��ʱ����д������ˮ����IJ�����
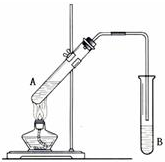 ���Թ�A�м���3mL �Ҵ���Ȼ������Թܱ���������2mL Ũ�����2mL ���ᣬ��ͼ��ʾ���Ӻ�װ����ȡ����������
���Թ�A�м���3mL �Ҵ���Ȼ������Թܱ���������2mL Ũ�����2mL ���ᣬ��ͼ��ʾ���Ӻ�װ����ȡ����������