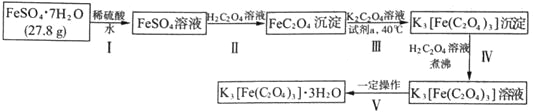
��Ŀ����
����Ŀ����һ�������£���3 mol A��1 mol B�����������ڹ̶��ݻ�Ϊ2 L���ܱ������У�������Ӧ��![]() ��2 minĩ�÷�Ӧ�ﵽƽ�⣬�������0.8 mol D��0.4 mol C�������жϲ���ȷ����
��2 minĩ�÷�Ӧ�ﵽƽ�⣬�������0.8 mol D��0.4 mol C�������жϲ���ȷ����
A.![]() =1
=1
B.2 minʱ��A��Ũ��Ϊ0.9 mol��L-1
C.2 minʱ��A��ƽ����Ӧ����Ϊ0.3 mol��L-1��min-1
D.2 minʱ��B��ƽ����Ӧ����Ϊ0.9 mol��L-1��min-1
���𰸡�D
��������
�����⽨����������ʽ��

A.�ɻ�ѧ������֮�ȵ��ڱ仯��֮�ȿɵã�![]() :2=0.4:0.8=1:2�����
:2=0.4:0.8=1:2�����![]() =1����A��ȷ��
=1����A��ȷ��
B.2minʱ��Ӧ�ﵽƽ�⣬A�����ʵ���Ϊ1.8mol��Ũ��![]() =0.9 mol�qL-1����B��ȷ��
=0.9 mol�qL-1����B��ȷ��
C.2min�ڣ�A�����ʵ����仯��Ϊ1.2mol����A��ƽ����Ӧ����Ϊ![]() =0.3 mol�qL-1�qmin-1����C��ȷ��
=0.3 mol�qL-1�qmin-1����C��ȷ��
D.�ɻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȿ�֪��2min�ڣ�![]() ����D����
����D����
��ѡD��

����Ŀ���� 100��ʱ���� 0.40 mol NO2 ������� 2 L ���ܱ������У��������·�Ӧ��2NO2(g)![]() N2O4(g) H < 0����ⷴӦ����������ݣ�
N2O4(g) H < 0����ⷴӦ����������ݣ�
ʱ��/s | 0 | 20 | 40 | 60 | 80 |
n(NO2)/mol | 0.40 | n1 | 0.26 | n3 | n4 |
n(N2O4)/mol | 0.00 | 0.05 | n2 | 0.08 | 0.08 |
����˵����ȷ����
A.0~20 s �ڣ�v(NO2) = 0.005 mol��L-1.s-1
B.��������Ӧ�� 120��ʱ���У���Ӧ�� 80 s ʱ��n(N2O4) < 0.08 mol
C.��������ʼʱ���� NO2 �������� 0.80 mol����ƽ��ʱ NO2 ת���ʽ�����
D.59 s ʱ��c(NO2)һ������ 0.12 mol��L-1