��Ŀ����
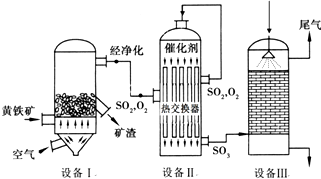
2���Ի�������Ҫ�ɷ���FeS2��Ϊԭ����������Ĺ�������ͼ��ͼ����1�����ݹ�������ͼ�ж�����˵������ȷ���ǣ�ѡ�������ĸ��DF��
A��Ϊʹ��������ȼ�գ��轫�����
B���������������SO2��ת����
C���ų��Ŀ����ɹ�����
D��ʹ�ô��������SO2�Ļ�ѧ��Ӧ���ʺ�ת����
E���豸I���ɵ����徭��������ҪĿ���Ƿ�ֹ�����ж�
F���Ƚ�������Ŀ����ʹSO2���������ݸ�SO3��������SO2��������SO3������
��2���豸�������Ϊ����¯��ȼ�ջ�����Ļ�ѧ����ʽΪ4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��
��3���豸��Ϊ�Ӵ��ң����ó�ѹ�������ø�ѹ��ԭ���dz�ѹʱSO2��ת�����Ѿ��ܸߣ����ø�ѹ��SO2��ת������߲���ȴ���������豸�ɱ����������У�Ϊ��ߴ���Ч�ʲ�ȡ�Ĵ�ʩ�о������塢�����¶���400��500�棻��������뷴Ӧ����ĽӴ�����ȣ�
��4���豸��Ϊ���������Ӷ�������98.3%����������SO3���õ��������ᣨ��Ũ���ᣩ������ˮ����SO3��Ϊ�˱������������������Ч�ʣ������������������ɹܣ�������������SO3��Ũ����ĽӴ��棬������SO3�����գ�
��5���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�SO2����Σ�SO2�ȿ���Ϊԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2�����ӷ���ʽ��SO2+Br2+2H2O=4H++2Br-+SO42-��
���� �Ի�������Ҫ�ɷ���FeS2��Ϊԭ����������Ĺ�������ͼ��
��һ����SO2����ȡ����Ҫԭ���ǻ�����Ϳ������ڸ��������գ�ʹ��Ԫ��ת���SO2���壬��Ӧ����ʽΪ��4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2���豸Ϊ����¯����������Ҫ���飬������Ϳ����ĽӴ��棻
�ڶ�����SO3����ȡ���ӷ���¯�г����������辭���������������Է�ֹ�����ж�������Ӵ����ڴ���������SO2��O2��Ӧ����SO3���豸Ϊ�Ӵ��ң�ͬʱ�漰�����Ľ��������������á�ƽ����ƶ������⣻
����������������ɣ��ӽӴ����г��������������������Ϊ���������γ�����ŨH2SO4���գ��Դ˽��1����2����
��3����ѹʱSO2��ת�����Ѿ��ܸߣ����ø�ѹ�����豸�ɱ�����ߴ���Ч�ʿ��Ծ������塢�����¶ȡ���������Ӵ������
��4��������������ˮ������������Ӧ�������γ�������ֹ��������������գ�Ũ����ķе�ߣ����������������γ�������ͬʱ��������������Ũ���ᣬ���������������ɹܣ���������������Ũ����ĽӴ��棬������������������գ�
��5���������������嵥�����õ��Ƕ�������Ļ�ԭ�Ժ��嵥�ʵ������ԣ�����������ԭ��Ӧ��д���ӷ���ʽ��
��� �⣺��1��A���ڷ���¯�У�ԭ�ϻ������ǹ��塢���������壬Ϊ�˼ӿ췴Ӧ���ʣ�����Ҫ����������飬����Ӵ��棬��߷�Ӧ���ʣ���A��ȷ��
B��ת����=��ת����ԭ�ϵ���/ԭ�ϵ�������100%���Ӵ����з�Ӧ2SO2+O2$?_{��}^{����}$2SO3��Ϊ���淴Ӧ�����ÿ�������Ŀ������������Ũ�ȣ���ʹƽ�������ƶ�������SO2�����ת����SO3������SO2ת������ߣ���B��ȷ��
C������¯�ų��Ŀ���ΪF2O3��F2O3�ɹ���������C��ȷ��
D������ֻ�ܸı䷴Ӧ���ʣ�����Ӱ��ת���ʣ���D����
E���豸I���ɵ����徭��������ҪĿ���Ƿ�ֹ�����ж�����E��ȷ��
F���Ƚ�������Ŀ����ʹSO2���������ݸ�SO3��������SO2����������������SO3�����գ���F����
�ʴ�Ϊ��DF��
��2���豸�������Ϊ����¯����Ӧ����ʽΪ��4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2���ʴ�Ϊ������¯��4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��
��3�����ó�ѹ�������ø�ѹ��ԭ���dz�ѹʱSO2��ת�����Ѿ��ܸߣ����ø�ѹ��SO2��ת������߲��࣬ȴ���������豸�ɱ����������У�Ϊ��ߴ���Ч�ʲ�ȡ�Ĵ�ʩ��ͨ���������塢�����¶���400��500�桢��������뷴Ӧ����ĽӴ����������ߴ���Ч�ʣ�
�ʴ�Ϊ����ѹʱSO2��ת�����Ѿ��ܸߣ����ø�ѹ��SO2��ת������߲���ȴ���������豸�ɱ����������塢�����¶���400��500�棻��������뷴Ӧ����ĽӴ�����ȣ�
��4����������SO3�����ˮ���գ�������Ӧ��SO3+H2O�TH2SO4���÷�ӦΪ���ȷ�Ӧ���ų����������������γɣ���������������ˮ֮�䣬�谭ˮ��������������գ���Ũ����ķе�ߣ����������������γ�������ͬʱ��������������Ũ���ᣬ���Թ�ҵ�ϴ���������������98.3%������������Һ�����յõ������̡�������������������ɹܣ���������������Ũ����ĽӴ��棬������������������գ�
�ʴ�Ϊ��98.3%������������ᣨ��Ũ���ᣩ���������������������Ч�ʣ�����SO3��Ũ����ĽӴ��棬������SO3�����գ�
��5��SO2����Br2�ķ�Ӧ�ж�����������Ϊ���ᣬ�嵥�ʱ���ԭΪ�廯�⣬���ӷ���ʽΪSO2+Br2+2H2O=4H++2Br-+SO42-���ʴ�Ϊ��SO2+Br2+2H2O=4H++2Br-+SO42-��
���� �����ǶԻ�ѧ�뼼������ҵ�����Ŀ��飬��Ҫѧ��ϸ����������ͼ�и����ʵı仯���н�𣬰����Ʊ�Ũ����Ĺ������̼��豸�����á������Ļ�ѧ��ӦΪ���Ĺؼ�����Ŀ�Ѷ��еȣ�

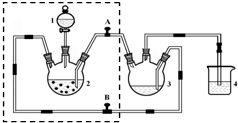
 �ɵ��ԭ��ʾ��ͼ��ͼ������ܷ�ӦΪ��Zn+2NH4+=Zn2++2NH3��+H2��������˵����ȷ���ǣ�������
�ɵ��ԭ��ʾ��ͼ��ͼ������ܷ�ӦΪ��Zn+2NH4+=Zn2++2NH3��+H2��������˵����ȷ���ǣ�������| A�� | ̼Ϊ��ص����� | |
| B�� | Zn���Ϸ�����ԭ��Ӧ | |
| C�� | ����п�̸ɵ��Ϊ���ε�� | |
| D�� | ��Ӧ2NH4++2e-=2NH3��+H2���ڸ����Ϸ��� |
��֪����ؽ������ӿ�ʼ��������ȫ����ʱ��pH��ΧΪ��
| ���� | Fe3+ | Fe2+ | Al3+ | Cu2+ |
| pH��Χ | 2.2��3.2 | 5.5��9.0 | 4.1��5.0 | 5.3��6.6 |
��1������H2O2�������ǽ�Fe2+����ΪFe3+����ʹ��ȡ��CuSO4•5H2O�����Ϊ������pH����Ӧ����5.0��
��2��д��H2O2��Fe2+���ӷ���ʽH2O2+2Fe2++2H+�T2Fe3++2H2O��
��3�����̢��м�������Al����������������Һ��Fe3+��H+��Ӧ����Al3+��
��4����������ѧ�Ļ�ѧ֪ʶ����AlCl3��Һ��������������ѧ�Լ����ܷ��Ƶ���ˮAlCl3���ܣ���ܻ��ܣ���ԭ����ֱ�Ӽ���AlCl3��Һ���ᷢ��ˮ�ⷴӦ�����յõ���������
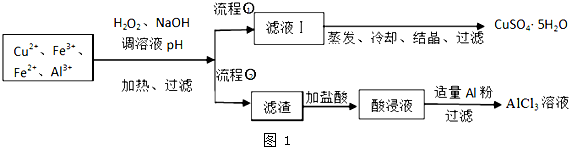
��5��ȡ���ΪV��L�������Һ�������еμ�a mol•L-1��NaOH��Һ�����ɳ��������ʵ��������ӵ�NaOH��Һ�������L����ϵ��ͼ2������V1��V2��V3��ʾ��ȡ�����Һ��n��Fe3+����n��Al3+��$\frac{4{V}_{2}-{V}_{1}-3{V}_{3}}{3��{V}_{3}-{V}_{2}��}$��
��֪�ױ����۵�Ϊ-95��C���е�Ϊ110.6��C���ӷ����ܶ�Ϊ0.866g/cm3����������۵�Ϊ122.4��C����25��C��95��C���ܽ�ȷֱ�Ϊ0.3g��6.9g��
���Ʊ���Ʒ����30.0mL�ױ���25.0mL1mol/L���������Һ��100��C�·�Ӧ30min��װ����ͼ1��ʾ��
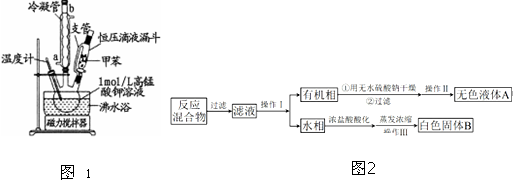
��1��ͼ�������ܵĽ�ˮ��Ϊa���a����b������֧�ܵ�������ƽ��ѹǿ�����ڼױ�˳������������ƿ��
��2���ڱ�ʵ���У�������ƿ����ʵ��ݻ���B������ĸ����
A��50mL B��100mL C��200mL D��250mL
������þƾ���ֱ�Ӽ��ȣ��÷�ˮԡ���ȵ��ŵ��DZ��ڿ����¶Ⱥ�ʹ�������Ⱦ��ȣ�
�������Ʒ����ͬѧ����������̷���ֲ�Ʒ������ͻ��ռױ�����ͼ2����
��3��������������Ƿ�Һ���������ʵIJ��ᆳ�������һ���ᴿ����ɫҺ��ױ���������������������
��4���ⶨ��ɫ������۵㣬��������115��C��ʼ�ۻ����ﵽ130��Cʱ�����������ۣ���ͬѧ�Ʋ��ɫ�����DZ�������KCl�Ļ�����������·��������ᴿ�ͼ��飬ʵ���������Ʋ���ȷ������ɱ������ݣ�
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ�������ˮ�У������ܽ⣬��ȴ�ᾧ�����ˣ� | �õ���ɫ�������ɫ��Һ | �v |
| �� | ȡ������Һ���Թ��У����������������ữ��AgNO3��Һ�� | ���ɰ�ɫ���� | ��Һ��Cl- |
| �� | �����ɫ���壬����ʹ���ۻ��������۵㣮 | ��ɫ������122.4������ʱ��ȫ�ۻ� | ��ɫ�����DZ����� |
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| �����mL�� | 24.00 | 24.10 | 22.40 | 23.90 |
| �ɷ� | Ũ��/��mol•L-1�� | �������� |
| HCl FeCl2 FeCl3 | -- 1.920 0.071 | 5.00% 8.94% 0.33% |
��2������ʯī���缫���������ϴ��Һʱ����ʼ�Σ������������������ɣ���ʱ��������йص������缫��ӦʽΪ2Cl--2e-=Cl2������������ϴ��Һ�м���KOH��Һ�кͺ��ں��ʵĵ�ѹ�µ�⣬��������������������������ɸ�����أ�K2FeO4����
��3������������ϴ��Һ��������ʯ����Ҫ�ɷ�ΪAl2O3��Fe2O3��SiO2���Լ����ƵĹ��ᣨ����ᣩ���Ʊ��۹����Ȼ����������������PAFSC�������巽����ͼ1��
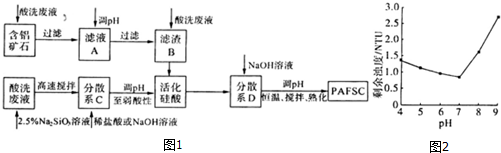
���ʵ�������ҺA��pH��Al3+��Fe3+ת��Ϊ������ԭ���ǵ�����ҺpH����Һ��[OH-]���Ӷ�ʹAl��OH��3��Fe��OH��3��Qc������Ksp�����������ҺpH����Һ��[OH-]���Ӷ�ʹAl��OH��3��Fe��OH��3�ij����ܽ�ƽ������������ƶ��������ó����ܽ�ƽ������۽��ͣ���
��PAFSC����������ˮ�Ĺ����У�Al3+���뷴Ӧ�����ӷ���ʽΪAl3++3H2O=Al��OH��3�����壩+3H+��
��25��ʱ��PAFSC�ij���Ч������ҺpH�ı仯��ͼ2��ʾ��ͼ�е�NTUΪ�Ƕȵ�λ������������pH��Χ�У�PAFSC����Ч����ѵ���b�������������ĸ����
a��4��5 b��5��7 c��7��8d��8��9
25��ʱ��pH��7����pH����PAFSC�ij���Ч�����Ա�ԭ���Ǽ�����ǿ��ʹ���巢���˾۳�����
| A�� | 5.0 m o l/L | B�� | 4.0 m o l/L | C�� | 4.5 m o l/L | D�� | 3.0 m o l/L |
| A�� | ����Ԫ�����ڱ���Ԫ�������ɵķ�����ͻ�����Ŀ�ѧ���ǰ����ӵ��� | |
| B�� | Ԫ�����ڱ��������ԭ��������С�����˳������ | |
| C�� | �ֳ�����Ԫ�����ڱ����߸����У���Ϊ�߸����ڣ���18�����У���Ϊ16���� | |
| D�� | ÿһ���ڶ��Ǵӽ���Ԫ�ؿ�ʼ���ǽ���Ԫ�ؽ��� |
 �����Ǹ�ˮ���[Cr��CH3COO��2��]2•2H2O�����ɫ���壩��һ���������ռ���ͨ���Զ�������Ӵ��ڣ���������ˮ���ѣ����ڴ������������ᣮʵ�����Ʊ������Ǹ�ˮ�����װ����ͼ��ʾ���漰�Ļ�ѧ����ʽ���£�
�����Ǹ�ˮ���[Cr��CH3COO��2��]2•2H2O�����ɫ���壩��һ���������ռ���ͨ���Զ�������Ӵ��ڣ���������ˮ���ѣ����ڴ������������ᣮʵ�����Ʊ������Ǹ�ˮ�����װ����ͼ��ʾ���漰�Ļ�ѧ����ʽ���£�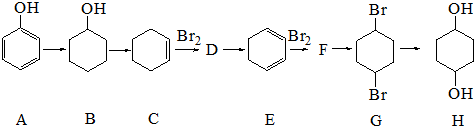
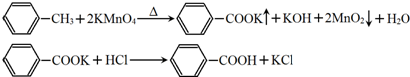
 +2NaOH$\stackrel{����}{��}$
+2NaOH$\stackrel{����}{��}$ +2NaBr+2H2O��
+2NaBr+2H2O��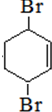 ��
�� +O2$��_{��}^{����}$2
+O2$��_{��}^{����}$2 +2H2O��
+2H2O�� ��
��