��Ŀ����
����Ŀ����Ҫ��ش��������⣺
��1��KMnO4��Ϊǿ������,������������Һ��������ǿ������,�����Խ����л�ԭ������Mn2���������Ի���Խ����л�ԭ������Ҫ��MnO2����д������������������H2O2�����ӷ���ʽ��_______________________��
��2����ҵ�Ͽ���KClO3��Һ��Na2SO3��Һ��ϡH2SO4�������Ƶ�ClO2���壬��д���÷�Ӧ�����ӷ���ʽ��_______________________��
��3����ǿ���Ի��ϡ����Һ�м���H2O2�����Խ���Һ��Ce3��������Ce(OH)4�������Է��룬��д���÷�Ӧ�����ӷ���ʽ________________________________________________��
��4��FeCl3��KClO��ǿ���������·�Ӧ������K2FeO4��KCl,д���÷�Ӧ�����ӷ���ʽ:____��
���𰸡�2MnO4��+ 5H2O2+6H��=2Mn2��+5O 2��+8H2O 2ClO3��+ SO32��+2H��= SO42��+ 2ClO2��+H2O 2Ce3��+ H2O2+ 6H2O=2Ce(OH)4��+6H�� 2Fe3++3ClO-+10OH-=2![]() +3Cl-+5H2O
+3Cl-+5H2O
��������
��1�������������£�KMnO4����H2O2����Mn2+����H2O2������ΪO2��
��2��KClO3��Һ��Na2SO3��Һ��ϡH2SO4�����·�����Ӧ������ClO2���壬ͬʱ����Na2SO4��
��3����ǿ���Ի��ϡ����Һ�У�H2O2���Խ���Һ��Ce3��������Ce(OH)4��������H2O2ת��Ϊˮ��OH-��
��4��FeCl3��KClO��ǿ���������·�Ӧ������K2FeO4��KCl����д����Ӧ����������������غ㷨������ƽ��
��1�������������£�KMnO4����H2O2������Mn2+��O2�����ӷ���ʽΪ2MnO4��+ 5H2O2+6H��=2Mn2��+5O 2��+8H2O����Ϊ��2MnO4��+ 5H2O2+6H��=2Mn2��+5O 2��+8H2O��
��2��KClO3��Һ��Na2SO3��Һ��ϡH2SO4�з�����Ӧ������ClO2�����Na2SO4�����ӷ���ʽΪ2ClO3��+ SO32��+2H��= SO42��+ 2ClO2��+H2O����Ϊ��2ClO3��+ SO32��+2H��= SO42��+ 2ClO2��+H2O��
��3��ǿ������Һ�У�H2O2��Ce3��������Ce(OH)4������H2O2����ԭΪˮ��OH-�����ӷ���ʽΪ2Ce3��+ H2O2+ 6H2O=2Ce(OH)4��+6H������Ϊ��2Ce3��+ H2O2+ 6H2O=2Ce(OH)4��+6H����
��4��FeCl3��KClO��ǿ���������·�Ӧ������K2FeO4��KCl�����ӷ���ʽΪ2Fe3++3ClO-+10OH-=2![]() +3Cl-+5H2O����Ϊ��2Fe3++3ClO-+10OH-=2
+3Cl-+5H2O������2Fe3++3ClO-+10OH-=2![]() +3Cl-+5H2O��
+3Cl-+5H2O��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�����з�Ӧ��mA(g)��nB(g)![]() pC(g)
pC(g)
��I�����ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
��1���÷�Ӧ���淴ӦΪ___�ȷ�Ӧ����m��n__p(������������������������)��
��2����B����ɫ���ʣ�A��C����ɫ�������C(�������)ʱ�������ɫ___����ά��������ѹǿ���䣬��������ʱ���������ɫ___��(����������������dz������������)��
��II�������ݻ��ɱ���ܱ������з�����Ӧ����һ���¶ȺͲ�ͬѹǿ�´ﵽƽ��ʱ���ֱ�õ�A�����ʵ���Ũ�����
ѹǿp/Pa | 2��105 | 5��105 | 1��106 |
c(A)/mol��L-1 | 0.08 | 0.20 | 0.44 |
��1����ѹǿ��2��105Pa���ӵ�5��105Paʱ��ƽ��___�ƶ�����������ң�������
��2��ά��ѹǿΪ2��105Pa������Ӧ�ﵽƽ��״̬ʱ����ϵ�й���amol���壬������ϵ�м���b molB�������´ﵽƽ��ʱ����ϵ�����������ʵ�����___mol��
��3����ѹǿΪ1��106Paʱ���˷�Ӧ��ƽ�ⳣ������ʽ��___��
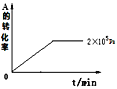
��4������������ͬʱ������������ѹǿ�·ֱ����÷�Ӧ��2��105Paʱ��A��ת������ʱ��仯��ͼ������ͼ�в��仭��ѹǿ�ֱ�Ϊ5��105Pa��1��106Paʱ��A��ת������ʱ��ı仯���ߣ�����ͼ���ϱ��ѹǿ����____
����Ŀ�����������(Na2S2O3)��һ�ֽⶾҩ�����ڷ�����顢����Ǧ����������ж����ٴ�����������ݡ���Ƥ�������Ȳ�֢.��������������Ի���Ի������ȶ�����������Һ�зֽ����S��SO2
ʵ��I��Na2S2O3���Ʊ�����ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2�Ƶã�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ��

(1)����a��������_______������b��������_______��b��������������Ϊ70%80%��H2SO4��Һ��Na2SO3���巴Ӧ�Ʊ�SO2��Ӧ�Ļ�ѧ����ʽΪ_______��c���Լ�Ϊ_______
(2)ʵ����Ҫ����SO2���������ʣ����Բ�ȡ�Ĵ�ʩ��_______ (д��һ��)
(3)Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ���SO2���ܹ�����ԭ����_______
ʵ���̽��Na2S2O3����������ӵ�������ԭ��Ӧ��
���ϣ�Fe3++3S2O32-Fe(S2O3)33-(�Ϻ�ɫ)
װ�� | �Լ�X | ʵ������ |
| Fe2(SO4)3��Һ | ��Ϻ���Һ�ȱ���Ϻ�ɫ��30s����Ϊ��ɫ |
(4)��������ʵ���������ж�����Fe3+��S2O32-��ԭΪFe2+��ͨ��_______(��������Լ�������)����һ��֤ʵ������Fe2+���ӻ�ѧ��Ӧ���ʺ�ƽ��ĽǶȽ���ʵ��������_______
ʵ��궨Na2S2O3��Һ��Ũ��
(5)��ȡһ�������IJ�Ʒ���Ƴ������������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ��÷�����ƽȷ��ȡ������K2Cr2O7(Ħ������Ϊ294gmol-1)0.5880g��ƽ���ֳ�3�ݣ��ֱ����3����ƿ�У���ˮ�����Һ�������������KI���ữ���������з�Ӧ��6I-+Cr2O72-+14H+ = 3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������ӦI2+2S2O32- = 2I- + S4O62-���������� Na2S2O3��Һ��ƽ�����Ϊ25.00 mL�������궨�������������Һ��Ũ��Ϊ_______molL-1