��Ŀ����
����Ŀ����������ͼ��ʾ���ó��Ľ�����ȷ����
A.ͼ����һ���¶��£����ں����ܱ������������Ի����µ�����������ʴ��������ϵѹǿ�ı仯���ߣ�����֪��ʼ��������Ҫ�������ⸯʴ
B.ͼ����ƽ����ϵ2NO2(g)![]() N2O4(g)����H����56.9 kJ��mol��1�ı�ijһ������v(��)��v(��)�ı仯���������֪t0ʱ�̸ı�������������¶�
N2O4(g)����H����56.9 kJ��mol��1�ı�ijһ������v(��)��v(��)�ı仯���������֪t0ʱ�̸ı�������������¶�
C.ͼ����ij�¶���c(CH3COOH)��c(CH3COO��)��0.100 mol��L��1�Ĵ���������ƻ����Һ��c(CH3COOH)��c(CH3COO��)��pH�Ĺ�ϵ������֪���¶���pH��3����Һ�У�Ka<10��4.75
D.ͼ����������2SO2��O2![]() 2SO3�ڴ��������·�Ӧ�����������ı仯���������֪�������Ĵ�Ч������
2SO3�ڴ��������·�Ӧ�����������ı仯���������֪�������Ĵ�Ч������
���𰸡�AC
��������
A. ͼ����һ���¶��£����ں����ܱ������������Ի����µ�����������ʴ��������ϵѹǿ�ı仯���ߣ����ݷ�Ӧ��ʼʱѹǿ������֪��ʼ��������Ҫ�������ⸯʴ������������࣬ѹǿ����ѡ��A��ȷ��
B. ͼ����ƽ����ϵ2NO2(g)![]() N2O4(g)����H����56.9 kJ��mol��1�ı�ijһ������v(��)��v(��)�ı仯�������t0ʱ�̸ı�������������¶ȣ������淴Ӧ���ʾ�������ͼ������Ӧ�����������ı�ʱ���䲻����ѡ��B����
N2O4(g)����H����56.9 kJ��mol��1�ı�ijһ������v(��)��v(��)�ı仯�������t0ʱ�̸ı�������������¶ȣ������淴Ӧ���ʾ�������ͼ������Ӧ�����������ı�ʱ���䲻����ѡ��B����
C. ͼ����ij�¶���c(CH3COOH)��c(CH3COO��)��0.100 mol��L��1�Ĵ���������ƻ����Һ��c(CH3COOH)��c(CH3COO��)��pH�Ĺ�ϵ������֪���¶���Ka=10��4.75���¶Ȳ���ʱKa���䣬��pH��3����Һ��Ka=10��4.75��ѡ��C��ȷ��
D. ͼ����������2SO2��O2![]() 2SO3�ڴ��������·�Ӧ�����������ı仯���������ͼ����Ϣ��֪������������Ӧ�Ļ�ܸ��ͣ���Ч�����ã�ѡ��D����
2SO3�ڴ��������·�Ӧ�����������ı仯���������ͼ����Ϣ��֪������������Ӧ�Ļ�ܸ��ͣ���Ч�����ã�ѡ��D����
��ѡAC��

 �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�����Ŀ��ij�о���ѧϰС��������롰�����仯����й����ʵ�ʵ��̽��������ͬ����������⣺
̽��һ�������ͼ��ʾװ�ý��С�����ˮ��Ӧ����ʵ��(�г�������)��

��1��Ӳ�ʲ�����B�з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________��
��2����ӦǰA��Ͷ�����Ƭ��Ŀ����____________________��
��3��װ��E�е�������__________________________________��
̽���� FeBr2��һ�ֻ���ɫ��Ƭ״�Ĺ��壬ij�о���ѧϰС��Ϊ��̽�����Ļ�ԭ�ԣ�����������ʵ�飺
I��ʵ����Ҫ90mL 0.10mol��L��1FeBr2��Һ
��1������FeBr2��Һ���ձ�����Ͳ������������ͷ�ι����IJ���������________��
��2�������й����ƹ�����˵����ȷ����________������ţ���
a����������ƽ��������Ϊ1.944g��FeBr2
b����������FeBr2��������ƿ�У���90mL����ˮ�ܽ�
c��ϴ���ܽ�FeBr2���ձ�������ϴ��Һת��������ƿ��
d������ƿ��ǩ�����õ�FeBr2��Һ
e������ʱ����������ƿ�̶���ʹ���Ƶ�FeBr2��ҺŨ��ƫ��
II��̽��FeBr2�Ļ�ԭ��
ȡ10mL����FeBr2��Һ�������еμ��������Ƶ���ˮ������Һ�ʻ�ɫ��ijͬѧ�Բ�����ɫ��ԭ������˼��裺
����1��Br����Cl2������Br2�ܽ�����Һ�У�
����2��Fe2+��Cl2������Fe3+��
��3����������±�����֤����
ʵ�鲽�衢Ԥ������ | ���� |
������Һ�м���__________�������ã� �����²�ʳȺ�ɫ���ϲ����ɫ | ����1��ȷ |
������Һ�м���_____________ ������Һ��Ϊ��ɫ | ����2��ȷ |
������1��ȷ����ʵ����з����Br2��ʵ�����������_______________ ��
��4����֪��Br2+2Fe2+=2Fe3++2Br-������50mL����FeBr2��Һ��ͨ���״��112mlCl2����Ӧ�����ӷ���ʽΪ__________________________________________��
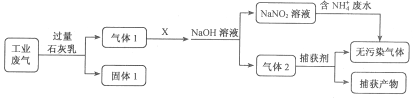
����Ŀ���Ȼ���ͭ�������л��ϳɹ�ҵ�еĴ�����Ϊ��ɫ���壬�������Ҵ�������ˮ�� ������Ũ�����γ�������ӣ�[CuCl2]�������ܳ�¶�ÿ�����Ѹ�������ɼ�ʽ�Ρ�ʵ���� �� ���������Ʊ���
ע��Na[CuCl2](���룬��Һ��ɫ)ˮNaCl+CuCl��(��ɫ����)����ش�
��1��д��ʵ������ȡNa[CuCl2]�����ӷ���ʽ_____________��
��2���жϲ���ڷ�Ӧ��ȫ��������_______________��
��3��������йس��˲���������˵����ȷ����_________��
A. ѡ�������Ҫ��Ϊ�˼ӿ�����ٶȣ��õ��ϸ���ij���
B. ������ƿ�ͳ�����֮��Ӧ����һ����ȫƿ������ƿӦ�밲ȫƿ�ij��������
C. ����ʱ���˹��˽�״����������������ֽ���γ�һ����ʵ�ij���
D. ϴ�ӳ���ʱ��Ӧ��Сˮ��ͷ��ʹϴ�Ӽ�����ͨ��������
��4����������Ҵ���ˮ��Һϴ�ӵ�Ŀ����______________��
��5��������������ո������н��У���ԭ����___________________��
��6���Ȼ���ͭ�Ķ���
��ȡ��Ʒ0.25g��10ml������FeCl3��Һ��250ml��ƿ�У�����ܽ⡣
����0.10mol��L��1��������[Ce(SO4)2]����Һ�ζ���
��֪��CuCl+FeCl3=CuCl2+FeCl2�� Fe2++Ce4+=Fe3++Ce3+
����ƽ�����������£�ƽ�����������ܳ���1%����
ƽ��������� | 1 | 2 | 3 |
0.25g��Ʒ�������������Һ�������ml�� | 24.35 | 24.05 | 23.95 |
����Ʒ��CuCl�Ĵ���Ϊ____________�������������λ��Ч���֣�