��Ŀ����
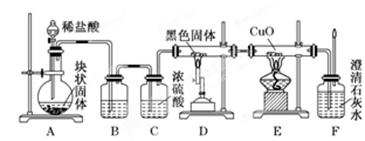
��17�֣���ѧ������3.0 mol��L-1ϡ������̽����ʵ�飬����ʱʵ����ֻ��18.4 mol��L-1Ũ���ᡣ����Ϊ������100 mL 3.0 mol��L-1ϡ���ᡣ
��1)�������ƹ������£�
�ټ�������Ũ���������� (ȷ��С�����һλ)����ȡŨ�������õ���Ͳ�Ĺ���� ����������ѡ��A.10 mL��B.25 mL��C.50 mL��D.100 mL����
��ϡ�͡�������������� ��
�۴��ϲ��õ���ϡ��Һ�����ȴ����������������ע�� ��������ˮϴ���ձ��ڱںͲ�����2-3�Σ�ϴ��ҺҲע�����С�����ҡ������ƿ��ʹ��Һ��Ͼ��ȡ�
�ܽ�����ˮע������ƿ����Һ��������ƿ�̶���1-2cm������ ��ˮ��Һ����̶������С��Ǻ�ƿ�����������µߵ���ҡ�ȡ�
�ݽ���õ���Һת�Ƶ��Լ�ƿ�У�����ͬѧʹ�á�
��2����������Һ�����У������������ʱ��ʵ��Ũ�Ȼ�����������ƫ�ߡ�ƫ�ͻ�Ӱ�죩
A.���õ�Ũ���᳤ʱ��������ܷⲻ�õ�������
B.����ƿ������ˮϴ�Ӻ������������ˮ
C.���ù����ձ���������δϴ�� ?
D.����ʱ���ӿ̶��� ?
��1)�������ƹ������£�
�ټ�������Ũ���������� (ȷ��С�����һλ)����ȡŨ�������õ���Ͳ�Ĺ���� ����������ѡ��A.10 mL��B.25 mL��C.50 mL��D.100 mL����
��ϡ�͡�������������� ��
�۴��ϲ��õ���ϡ��Һ�����ȴ����������������ע�� ��������ˮϴ���ձ��ڱںͲ�����2-3�Σ�ϴ��ҺҲע�����С�����ҡ������ƿ��ʹ��Һ��Ͼ��ȡ�
�ܽ�����ˮע������ƿ����Һ��������ƿ�̶���1-2cm������ ��ˮ��Һ����̶������С��Ǻ�ƿ�����������µߵ���ҡ�ȡ�
�ݽ���õ���Һת�Ƶ��Լ�ƿ�У�����ͬѧʹ�á�
��2����������Һ�����У������������ʱ��ʵ��Ũ�Ȼ�����������ƫ�ߡ�ƫ�ͻ�Ӱ�죩
A.���õ�Ũ���᳤ʱ��������ܷⲻ�õ�������
B.����ƿ������ˮϴ�Ӻ������������ˮ
C.���ù����ձ���������δϴ�� ?
D.����ʱ���ӿ̶��� ?
16.3mL B��25mL��1�֣�����ÿ��2�֣� ��װ��ˮ���ձ��ڱڻ���ע��Ũ���ᣬ�ӱ߽��� 100mL����ƿ ��ͷ�ι� ƫ�� ��Ӱ�� ƫ�� ƫ��
�����������1��1����Ũ��������ΪV��18.4mol/L��V=0.1L��3.0mol/L��V=0.0163L=16.3mL��ѡȡ��Ͳ���ݻ�Ӧ�Դ��ڻ������ȡ��Һ���������ѡB��
�ʴ�Ϊ��16.3ml��B��
Ȼ��Ũ�������ձ��ڱڻ��������ձ��У����ò��������裬��ֹҺ��ɽ���
�ʴ�Ϊ����װ��ˮ���ձ��ڱڻ���ע��Ũ���ᣬ�ӱ߽��裻
3��ϴ��ҺҪת�Ƶ�100mL����ƿ�У��ʴ�Ϊ��100mL����ƿ��
4��������ˮע������ƿ����Һ��������ƿ�̶���1-2cm������ ��ͷ�ιܼ�ˮ��Һ����̶������У��Ǻ�ƿ�����������µߵ���ҡ�ȡ�
�ʴ�Ϊ����ͷ�ιܣ�
��2��A�����õ�Ũ���᳤ʱ��������ܷⲻ�õ������У�Ũ��������ˮ�ԣ��������ʵ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ�
B������ƿ������ϴ�Ӻ������������ˮ����Ӱ�����ʵ����ʵ�������Һ�����������������Һ�����ʵ���Ũ����Ӱ�졣
C�����ù����ձ���������δϴ�ӣ��������ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
D������ʱ������Һ��Һ���棬������Һ�����ƫС������������Һ��Ũ��ƫ�ߡ�
�ʴ�Ϊ��ƫ�ͣ���Ӱ�죻ƫ�ͣ�ƫ�ߣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ��������ȡŨ��������ѡ����Ͳ

��ϰ��ϵ�д�
�����Ŀ

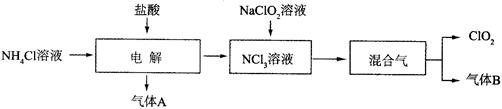
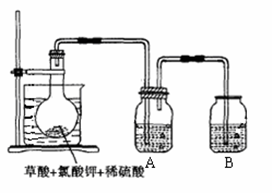
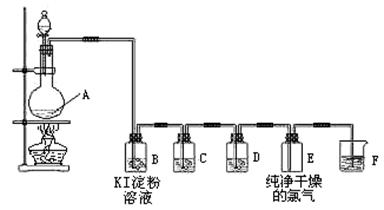
 MnCl2+C12��+2H2O���ڷ���ʽ�ϱ������ת�Ƶķ������Ŀ���÷�Ӧ���������� ��
MnCl2+C12��+2H2O���ڷ���ʽ�ϱ������ת�Ƶķ������Ŀ���÷�Ӧ���������� ��