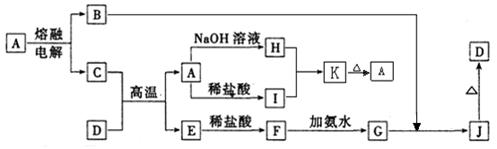
��Ŀ����
��������һ����Ҫ�ķ��Σ���Ҫ����ũҵɱ������ɱ�����ľ�ķ�������ʵ���ҿ�ͨ����ͼ��ʾ�������Է����ᣨH2SiF6��������Ϊԭ����ȡ�����ƣ����õ�����Ʒ�Ȼ�泥�

�й�������ˮ���ܽ�ȷֱ�Ϊ��
NH4Cl��10��ʱ33.3g��20��ʱ37.2g��30��ʱ41.4g��
NaF��20��ʱ4g��Na2SiF6���ܡ�
��ش��������⣺
��1��ָ������Ʒ����;�� ��������һ�ּ��ɣ�
��2����������Ҫ�õ��IJ�����������������©���⣬���� ___ ��
��3������II�������� ��
��4��������ľ�������� �� ��
��5�����������з���������ѧ��Ӧ����ֱ�д���仯ѧ����ʽ��
��һ���� ��
�ڶ����� ��
��6��������NH4HCO3�����������ԭ���� ��

�й�������ˮ���ܽ�ȷֱ�Ϊ��
NH4Cl��10��ʱ33.3g��20��ʱ37.2g��30��ʱ41.4g��
NaF��20��ʱ4g��Na2SiF6���ܡ�
��ش��������⣺
��1��ָ������Ʒ����;�� ��������һ�ּ��ɣ�
��2����������Ҫ�õ��IJ�����������������©���⣬���� ___ ��
��3������II�������� ��
��4��������ľ�������� �� ��
��5�����������з���������ѧ��Ӧ����ֱ�д���仯ѧ����ʽ��
��һ���� ��
�ڶ����� ��
��6��������NH4HCO3�����������ԭ���� ��
��11�֣�
��1�������� (1��)
��2���ձ� (1��)
��3���ؽᾧ (1��)
��4������Ũ�������½ᾧ (��1��)
��5��6NH4HCO3 + H2SiF6 ="=" 6NH4F + H2SiO3�� + 6CO2��+ 3H2O
NH4F + NaCl ="=" NaF�� + NH4Cl (��2��)
��6����֤H2SiF6��Ӧ���ף�����������NaFʱ�������϶�Na2SiF6���ʣ�2�֣�
��1�������� (1��)
��2���ձ� (1��)
��3���ؽᾧ (1��)
��4������Ũ�������½ᾧ (��1��)
��5��6NH4HCO3 + H2SiF6 ="=" 6NH4F + H2SiO3�� + 6CO2��+ 3H2O
NH4F + NaCl ="=" NaF�� + NH4Cl (��2��)
��6����֤H2SiF6��Ӧ���ף�����������NaFʱ�������϶�Na2SiF6���ʣ�2�֣�
�����������2���������ǹ��˲������õ��IJ������������ձ�����������©�����ʴ�Ϊ���ձ�����3���������ǽ���NaFϴ�ӳ�ȥ���������ʣ��Եõ�������NaF�������Ϊ�ؽᾧ����4���������ǽ���Һ�е����ʽ�һ����ȡ���ʲ����Ǽ��������ܼ������������������ȴ���ʴ�Ϊ����ȥNaF��NH4Cl ��������ʣ�����Ũ����Һ�����������������ȴ��
��5����һ����Ӧ�ķ���ʽ����Ӧ����H2SiF6����NH4HCO3����������H2SiO3��CO2�����������غ㻹Ӧ��NH4F����Ӧ�Ļ�ѧ����ʽΪH2SiF6+6NH4HCO3=6NH4F+H2SiO3��+6CO2��+3H2O���ڶ�����Ӧ�ǽ���һ�����ɵ�NH4Fת��ΪNaF������ķ�Ӧ��ΪNaCl����������������Һ���ܽ��ԽС���������������ԣ�����NaF��NaCl���ܽ���С����Ӧ�Ļ�ѧ����ʽΪ
NH4F+NaCl=NH4Cl+NaF����
�ʴ�Ϊ��H2SiF6+6NH4HCO3=6NH4F+H2SiO3��+6CO2��+3H2O��NH4F+NaCl=NH4Cl+NaF������6��������NH4HCO3�����������ָ��һ�������б��뱣֤H2SiF6��ȫ����Ӧ���Է�ֹ����Na2SiF6������Ӱ����ȡ���ʵĴ��ȣ�
�ʴ�Ϊ����֤H2SiF6��ȫ����Ӧ����ֹ�ڼ���NaCl��H2SiF6��NaCl��Ӧ����Na2SiF6��������NaF��Ӱ��NaF���ȡ�
���������⿼���Ϊ�ۺϣ���Ŀ�ѶȽǶȣ�ע������Ʊ����̵ķ�Ӧԭ��������������Ϣ��𣬱����״���Ϊ��ػ�ѧ����ʽ����д��ע��������غ�ĽǶ�˼����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


 X+CO2+H2O ��Z+CO2
X+CO2+H2O ��Z+CO2 X+O2 ��Z+H2O
X+O2 ��Z+H2O
 ����ʾ��4FeS2+11O2
����ʾ��4FeS2+11O2 2Fe2O3+8SO2��
2Fe2O3+8SO2��