题目内容
【题目】部分难溶物的颜色和常温下的Ksp如下表所示:
Cu(OH)2 | CuOH | CuCl | Cu2O | |
颜色 | 蓝色 | 黄色 | 白色 | 砖红色 |
Ksp(25 ℃) | 1.6×10-19 | 1.0×10-14 | 1.2×10-6 | — |
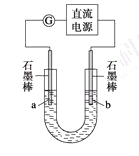
某研究性学习小组对电解食盐水进行了如下探究:
实验Ⅰ装置如图所示,接通电源后,发现a、b电极上均有气泡产生。
(1)电解过程中的总离子反应方程式为_________________________________________。
(2)为了确定电源的正、负极,下列操作一定行之有效的是_______。
A.观察两极产生气体的颜色
B.往U形管两端分别滴入数滴酚酞试液
C.用燃着的木条靠近U形管口
D.在U形管口置一张湿润的淀粉KI试纸
实验Ⅱ把上述电解装置的石墨棒换成铜棒,用直流电源进行电解,装置如图所示。
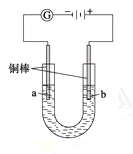
观察到的现象如下所示:
①开始无明显现象,随后液面以下的铜棒表面逐渐变暗;
②5 min后,b极附近开始出现白色沉淀,并逐渐增多,且向a极扩散;
③10 min后,最靠近a极的白色沉淀开始变成红色;
④12 min后,b极附近的白色沉淀开始变成黄色,然后逐渐变成橙黄色;
⑤a极一直有大量气泡产生;
⑥停止电解,将U形管中悬浊液静置一段时间后,上层溶液呈无色,没有出现蓝色,下层沉淀全部显砖红色。
(3) a极发生的电极反应方程式为________________________________________________________。
(4) 电解5 min后,b极发生的电极反应方程式为___________________________________________。
(5)12 min后,b极附近出现的橙黄色沉淀的成分是_____,原因是___________________________________________________________________________________。
【答案】2Cl-+2H2O![]() 2OH-+H2↑+Cl2↑ BD 2H++2e-= H2↑ (或2H2O+2e-=2OH-+H2↑) Cu+Cl--e-=CuCl↓ CuOH和Cu2O Ksp(CuOH)<Ksp(CuCl),CuCl转化为黄色的CuOH沉淀,CuOH不稳定分解生成Cu2O,所以橙黄色沉淀的成分为CuOH和Cu2O的混合物
2OH-+H2↑+Cl2↑ BD 2H++2e-= H2↑ (或2H2O+2e-=2OH-+H2↑) Cu+Cl--e-=CuCl↓ CuOH和Cu2O Ksp(CuOH)<Ksp(CuCl),CuCl转化为黄色的CuOH沉淀,CuOH不稳定分解生成Cu2O,所以橙黄色沉淀的成分为CuOH和Cu2O的混合物
【解析】
(1)该装置中电极都是惰性电极,阳极上氯离子放电生成氯气、阴极上水得电子生成氢气同时生成氢氧根离子;
(2)在电解氯化钠的装置中,和电源正极相连的是阳极,该极上会产生氯气,和负极相连的是阴极,该极上产生的是氢氧化钠和氢气,根据产物的性质来判断电极;
(3)在电解池的阴极上是阳离子发生得电子的还原反应;
(4)根据题意:电解5min后会产生白色沉淀来回答;
(5)根据表中物质的颜色,CuOH是黄色,但是其不稳定,易分解为砖红色的氧化亚铜。
(1)电解饱和食盐水得到的产物是氢氧化钠、氯气和氢气,离子方程式:2Cl-+2H2O![]() 2OH-+H2↑+Cl2↑;
2OH-+H2↑+Cl2↑;
故答案为:2Cl-+2H2O![]() 2OH-+H2↑+Cl2↑;
2OH-+H2↑+Cl2↑;
(2)电解氯化钠的装置中,和电源正极相连的是阳极,该极上会产生氯气,和负极相连的是阴极,该极上产生的是氢氧化钠和氢气,
A.电解池的阳极上可以产生氯气等有颜色的气体,还可以产生氧气等无色气体,故观察两极产生气体的颜色不可以判断正负极,故A错误;
B.往U形管两端分别滴入数滴酚酞试液,变红色的即是阴极,对应的是电源的负极,故B正确;
C.用燃着的木条靠近U形管口,只能检验有氢气或是氧气产生时的电极反应,但是对其他物质的产生无法检验,故C错误;
D.在U形管口置一张湿润的淀粉KI试纸,能使湿润的淀粉KI试纸变蓝证明氯气的产生,该电极是阳极,对应的是电源的正极,故D正确;
故选BD;
(3)a极是阴极,在电解池的阴极上是水中的阳离子氢离子发生得电子的还原反应:2H++2e-=H2↑(或2H2O+2e-═2OH-+H2↑);
故答案为:2H++2e-=H2↑(或2H2O+2e-═2OH-+H2↑);
(4)根据题意知道电解5min后,b极产生的白色沉淀,从表中知道,该物质是CuCl,所以该极上发生的电极反应方程式为Cu+Cl--e-═CuCl↓;
故答案为:Cu+Cl--e-═CuCl↓;
(5)根据题意结合表中物质的颜色知道:12min后,b极附近出现的橙黄色沉淀的成分是CuOH和Cu2O,因为Ksp(CuOH)<Ksp(CuCl),所以电解过程中CuCl转化为黄色的CuOH沉淀,CuOH不稳定分解生成Cu2O,导致物质的颜色为橙色;
故答案为:CuOH和Cu2O;Ksp(CuOH)<Ksp(CuCl),CuCl转化为黄色的CuOH沉淀,CuOH不稳定分解生成Cu2O,所以橙黄色沉淀的成分为CuOH和Cu2O的混合物。

【题目】一定条件下,可逆反应2A(g)![]() B(g)+3C(g),反应处于平衡状态的是( )
B(g)+3C(g),反应处于平衡状态的是( )
选项 | 正反应速率 | 逆反应速率 |
A | v(A)=2mol·L-1·min-1 | v(B)=2mol·L-1·min-1 |
B | v(A)=2mol·L-1·min-1 | v(C)=2mol·L-1·min-1 |
C | v(A)=1mol·L-1·min-1 | v(B)=2mol·L-1·min-1 |
D | v(A)=1mol·L-1·min-1 | v(C)=1.5mol·L-1·min-1 |
A.AB.BC.CD.D
【题目】(1)某学习小组利用下图装置制取氯气并探究其性质。
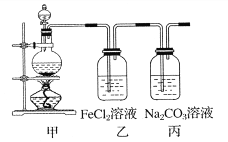
①甲装置中反应的化学方程式是_____________________________________;
②证明乙装置中FeCl2溶液与Cl2发生了反应的实验方法是(只注明试剂、现象)__________________________________________________________;
③丙装置中通入少量Cl2,可制得某种生活中常用的漂白、消毒的物质。已知碳酸的酸性强于次氯酸,则丙中反应的化学方程式是____________________________________。
(2)有一瓶长期放置的漂白粉,请利用以下仪器和试剂,完成该漂白粉成份的探究。
试管、胶头滴管、带导管的单孔塞、蒸馏水、1mol·L-1盐酸、品红溶液、新制澄清石灰水。
(提出假设)假设一:该漂白粉未变质,含CaCl2、Ca(ClO)2;
假设二:该漂白粉全部变质,含________________;
假设三:该漂白粉部分变质,含CaCl2、Ca(ClO)2、CaCO3 。
(进行实验)在答题卡上完成下表(不必检验Ca2+、Cl-):
实验步骤 | 预期现象和结论 | |
① | 用A试管取少量澄清石灰水备用,用B试管取少量样品,再向B试管___________________________ | 若无气体放出且澄清石灰水未见浑浊,则假设一成立;______________________________ |
② | __________________________ | ___________________________________________ |