��Ŀ����
��13�֣�a��gԪ�ص�ԭ������㶼��M�㣬�������������£�
|
Ԫ �� |
a |
b |
c |
d |
e |
f |
g |
|
���������� |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
�û�ѧ��������������⣺
��1��Ԫ��b��Ԫ��g�γɵ��ȶ�����������ʽ��
��2����d��e��f��g���γɵ���̬�⻯�����ȶ��������ǣ�
��3����ҵ�Ʊ�����d�Ļ�ѧ��Ӧ����ʽ��
��4��Ԫ��c������������Ӧ��ˮ����ֱ���Ԫ��a��f������������Ӧ��ˮ���ﷴӦ�����ӷ���ʽ����
��
��ϰ��ϵ�д�
�����Ŀ
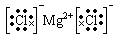 ��2�֣�
��2�֣�
 Si+2CO ��3�֣�
Si+2CO ��3�֣�






 2EB2(g)+O2(g)
2EB2(g)+O2(g)  2EB3(g);��H=-197kJ/mol ��������̶������룬��x=1����ƽ��ʱ���ҵ�ѹǿ����ʼʱ��0.7���������ҷ�Ӧ��ƽ�ⳣ��K=_______________��
2EB3(g);��H=-197kJ/mol ��������̶������룬��x=1����ƽ��ʱ���ҵ�ѹǿ����ʼʱ��0.7���������ҷ�Ӧ��ƽ�ⳣ��K=_______________��