��Ŀ����
��������Ҫ�Ļ�����Ʒ���ڻ�����ѧʵ���У�������Ҫ�����ã�
��1�����ʵ���Ũ��Ϊ18.4mol/L����������Ϊ0.98��Ũ��������ˮ����ʱ���������������½���0.87���ܶ�1.8g?cm-3������ʱ����ʧȥ���������� ��������Ϊ0.87����������ʵ���Ũ��Ϊ______��������λС������ͬ����50mL 18.4mol/L��Ũ������Ϊ�����ʱ��������ˮ______g��
��2����ҵ���Ը����������ᡢ����Ϊԭ����ȡ�����[NH4Al��SO4��2?12H2O]������������Ӧԭ�����£����Ը����������ɷ�������ķ�Ӧ����
Al2O3+3H2SO4��Al2��SO4��3+3H2O����
Al2��SO4��3+H2SO4+2NH3��2NH4Al��SO4��2 ����
ij������ͬʱ��ȡ���������������ͨ����������������������ֲ�Ʒ�IJ���������ʹ�Ƶõ�������������������ʵ���֮��Ϊ1��1����Ͷ��ʱ����������������ʵ���֮����______
��3�����Ṥҵ�ϴ���ýӴ��������ᣨ��������������������Ϊ20%����Ϊʹ���������ճ�֣���ͨ�����40%�Ŀ����������պ�¯����SO2���������Ϊ______��
��4��������¯��������������ֱ������Ӵ��ң��������������5%��ͬ��ͬѹ�²ⶨ�����Լ���SO2��ת���ʣ�
�⣺��1����������Ϊ0.87���ܶ�Ϊ1.8g?cm-3��Ũ��������ʵ���Ũ��Ϊ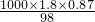 mol/L=15.98 mol?L-1��
mol/L=15.98 mol?L-1��
50mL 18.4mol/L��Ũ���������Ϊ��50mL��1.84g?cm-3=92g�����ˮ������ΪXg��
92g��0.98=��92+X��g��0.87����ã�X=11.63��
�ʴ�Ϊ��15.98��11.63��
��2�����Ʊ���[NH4Al��SO4��2?12H2O��Al2��SO4��3�����ʵ��������ʵ�����Ϊ1mol���������غ�ĽǶȷ�������n��Al��=1mol+2mol=3mol��n��SO42-��=2mol+3mol=5mol�����ԣ�n��Al2O3��=1.5mol��n��H2SO4��=5mol��n��Al2O3����n��H2SO4��=1.5mol��5mol=3��10��
�ʴ�Ϊ��3��10��
��3����FeS2Ϊ1 mol����ȫ������Ҫ��n��O2��=2.75 mol��n��SO2��=2mol������40%�������Ϊ��n��������=2.75��0.2��1.4=19.25 mol������������������Ϊ19.25mol��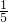 -2.75mol=1.1mol�����������ʵ���Ϊ19.25mol��
-2.75mol=1.1mol�����������ʵ���Ϊ19.25mol��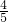 =15.4mol��SO2Ϊ2mol��SO2��������������ʵ�������Ϊ��
=15.4mol��SO2Ϊ2mol��SO2��������������ʵ�������Ϊ��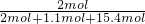 =0.11��
=0.11��
�ʴ�Ϊ��0.11��
��4����SO2��ת����ΪY��
2SO2 +O2 =2SO3 ������١�V
2 1
2mol��Y 1mol��Y
��Ӧǰ������ܵ����ʵ���Ϊ��18.5mol���������������5%�������ʵ�������5%����18.5mol��5%=1mol��Y�����Y=9.25����SO2��ת����93%��
�ʴ�Ϊ��93%��
��������1������c=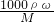 ������������Ϊ0.87��Ũ��������ʵ���Ũ�ȣ�������ˮǰ�����ʵ��������������
������������Ϊ0.87��Ũ��������ʵ���Ũ�ȣ�������ˮǰ�����ʵ��������������
��2�����������غ㶨����ʽ���㣻
��3����FeS2Ϊ1 mol�����ݷ���ʽ������ĵ����������ɵ�SO2��Ȼ�������Ӧ��������ijɷ��Լ����ʵ�����������SO2�����������
��4�����ݻ�ѧ����ʽ2SO2+O2=2SO3�����㣮
������������Ҫ�����˻�ѧ���㣬ץסϰ���з����Ļ�ѧ��Ӧ�ǽ���Ĺؼ���ע�����֪ʶ�Ļ��ۣ���Ŀ�Ѷ��еȣ�
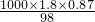 mol/L=15.98 mol?L-1��
mol/L=15.98 mol?L-1��50mL 18.4mol/L��Ũ���������Ϊ��50mL��1.84g?cm-3=92g�����ˮ������ΪXg��
92g��0.98=��92+X��g��0.87����ã�X=11.63��
�ʴ�Ϊ��15.98��11.63��
��2�����Ʊ���[NH4Al��SO4��2?12H2O��Al2��SO4��3�����ʵ��������ʵ�����Ϊ1mol���������غ�ĽǶȷ�������n��Al��=1mol+2mol=3mol��n��SO42-��=2mol+3mol=5mol�����ԣ�n��Al2O3��=1.5mol��n��H2SO4��=5mol��n��Al2O3����n��H2SO4��=1.5mol��5mol=3��10��
�ʴ�Ϊ��3��10��
��3����FeS2Ϊ1 mol����ȫ������Ҫ��n��O2��=2.75 mol��n��SO2��=2mol������40%�������Ϊ��n��������=2.75��0.2��1.4=19.25 mol������������������Ϊ19.25mol��
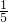 -2.75mol=1.1mol�����������ʵ���Ϊ19.25mol��
-2.75mol=1.1mol�����������ʵ���Ϊ19.25mol��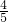 =15.4mol��SO2Ϊ2mol��SO2��������������ʵ�������Ϊ��
=15.4mol��SO2Ϊ2mol��SO2��������������ʵ�������Ϊ��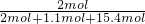 =0.11��
=0.11���ʴ�Ϊ��0.11��
��4����SO2��ת����ΪY��
2SO2 +O2 =2SO3 ������١�V
2 1
2mol��Y 1mol��Y
��Ӧǰ������ܵ����ʵ���Ϊ��18.5mol���������������5%�������ʵ�������5%����18.5mol��5%=1mol��Y�����Y=9.25����SO2��ת����93%��
�ʴ�Ϊ��93%��
��������1������c=
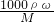 ������������Ϊ0.87��Ũ��������ʵ���Ũ�ȣ�������ˮǰ�����ʵ��������������
������������Ϊ0.87��Ũ��������ʵ���Ũ�ȣ�������ˮǰ�����ʵ����������������2�����������غ㶨����ʽ���㣻
��3����FeS2Ϊ1 mol�����ݷ���ʽ������ĵ����������ɵ�SO2��Ȼ�������Ӧ��������ijɷ��Լ����ʵ�����������SO2�����������
��4�����ݻ�ѧ����ʽ2SO2+O2=2SO3�����㣮
������������Ҫ�����˻�ѧ���㣬ץסϰ���з����Ļ�ѧ��Ӧ�ǽ���Ĺؼ���ע�����֪ʶ�Ļ��ۣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ
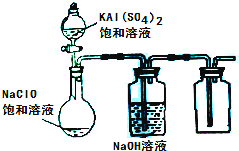 ��2013?����ģ�⣩NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
��2013?����ģ�⣩NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��