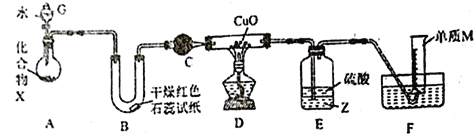
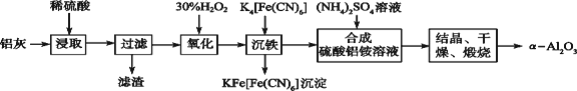
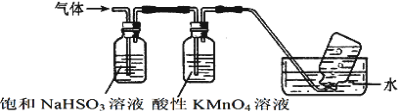
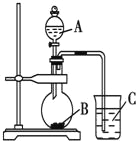
��Ŀ����
����Ŀ��ij�л���A����Է�������Ϊ62��Ϊ��һ���ⶨA �Ļ�ѧʽ��ȡ3.1g A��ȫȼ�գ��õ�������̼��ˮ�������������Ⱥ�ͨ��������Ũ����ͼ�ʯ�ң����߷ֱ�����2.7 g��4.4 g������ÿ����Ӧ��ȫ����
��1�����л����ʵ��ʽ��__________________������ʽ��________________��
��2�����������ʾ�С�C��C�����͡�O��H�����������գ����˴Ź�������ֻ��2�����շ��ҷ����֮��Ϊ1��2���ƶϸ��л���Ľṹ��ʽ��__________________��
��3�����л���������Ʒ�Ӧ�Ļ�ѧ����ʽ��____________________��
���𰸡� CH3O C2H6O2 CH2OHCH2OH CH2OHCH2OH+2Na![]() CH2ONaCH2ONa+H2
CH2ONaCH2ONa+H2
���������������������Ⱥ�ͨ��������Ũ����ͼ�ʯ�ң����߷ֱ�����2.7 g��4.4 g�����ӵ������ֱ���ˮ�Ͷ�����̼�������������л���A�����ԭ��������������غ㶨���ж�ʵ���ҡ�����ʽ�������л����������д�ṹ��ʽ��
��⣺��1��3.1gA�����ʵ�����0.05mol�����ɵ�ˮ��2.7g�����ʵ�����2.7g��18g/mol��0.15mol��������ԭ�ӵ����ʵ�����0.3mol�����ɵ�CO2��4.4g�����ʵ�����4.4g��44g/mol��0.1mol������̼ԭ�ӵ����ʵ�����0.1mol�����Ը���ԭ���غ��֪��A�����к��е�̼����ԭ�Ӹ����ֱ���2����6��������ԭ�ӵĸ�����![]() ��������A�Ļ�ѧʽ��C2H6O2�����ʽ��CH3O��
��������A�Ļ�ѧʽ��C2H6O2�����ʽ��CH3O��
��2�����ݺ��������ʾ�С�C��C�����͡�O��H�����������գ��˴Ź�������ֻ��2�����շ��ҷ����֮��Ϊ1��2��֪�����л������Ҷ������ṹ��ʽ��HOCH2CH2OH��
��3�����ܺͽ����Ʒ�Ӧ������������÷�Ӧ�Ļ�ѧ����ʽ��HOCH2CH2OH��2Na��NaOCH2CH2ONa��H2����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�