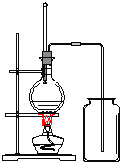
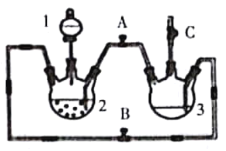
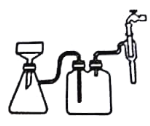
��Ŀ����
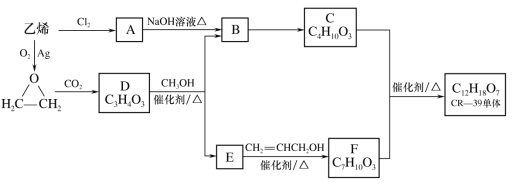
����Ŀ������������Ҫ�������Ƹ�Ѫѹ���Ѫ������˥�ߣ���ϳ�·�����£�

��֪������ ![]()
����DCC��һ�ֺ�ǿ����ˮ����
(1)������A��������___________________��
(2)B��C������Լ���������________________��
(3)C��D�Ļ�ѧ����ʽ��____________________��
(4)D��E�ķ�Ӧ������______________________��
(5)F�Ľṹ��ʽ��____________________________��
(6)K���ڻ����칹��K����G��������·�ߺϳɣ�

��X������Ԫ����̼̼˫������ṹ��ʽ��______��
�������ϳ�����������·���У���Yת��ΪG������F��Ӧ������ֱ����Y����ҪĿ����______����ϳ�·����______________����ĸ����ת��Ŀ����ͬ��
���𰸡��ױ� NaOH��ˮ��Һ���� ![]() ȡ����Ӧ
ȡ����Ӧ ![]()
![]() ����Y�е��Ȼ� C��D
����Y�е��Ȼ� C��D
��������
�����ϳɷ�������H��Pd/C��������H2������֪��Ϣ���ķ�Ӧ������H�����������ķ���ʽ���ɵ�H�Ľṹ��ʽΪ ��DCC��һ�ֺ�ǿ����ˮ����F��G������ˮ��Ӧ����H�����G��F�ķ���ʽ��H�Ľṹʽ������֪F�Ľṹ��ʽΪ
��DCC��һ�ֺ�ǿ����ˮ����F��G������ˮ��Ӧ����H�����G��F�ķ���ʽ��H�Ľṹʽ������֪F�Ľṹ��ʽΪ ��G�Ľṹ��ʽΪ
��G�Ľṹ��ʽΪ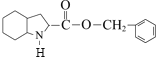 ��E��Pd/C��������H2������֪��Ϣ���ķ�Ӧ������F�Ľṹʽ������ʽ��E�ķ���ʽ��E�Ľṹ��ʽΪ
��E��Pd/C��������H2������֪��Ϣ���ķ�Ӧ������F�Ľṹʽ������ʽ��E�ķ���ʽ��E�Ľṹ��ʽΪ �����D��E�ķ���ʽ��E�ṹʽ��D��
�����D��E�ķ���ʽ��E�ṹʽ��D��![]() ��һ�������·���ȡ����Ӧ����E��D�Ľṹ��ʽΪ
��һ�������·���ȡ����Ӧ����E��D�Ľṹ��ʽΪ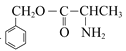 ��C��
��C��![]() �ڴ���������������D����Ϸ���ʽ��C�Ľṹ��ʽΪ
�ڴ���������������D����Ϸ���ʽ��C�Ľṹ��ʽΪ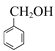 ��B��һ�������·���±������ˮ������C����B�Ľṹ��ʽΪ
��B��һ�������·���±������ˮ������C����B�Ľṹ��ʽΪ![]() ��A�ڹ��յ���������Cl2����ȡ����Ӧ����B��A�Ľṹ��ʽΪ
��A�ڹ��յ���������Cl2����ȡ����Ӧ����B��A�Ľṹ��ʽΪ![]() ���ݴ˷������
���ݴ˷������
(1)���ݷ���������A�Ľṹ��ʽΪ![]() �������Ǽױ���
�������Ǽױ���
(2)���ݷ�����B��һ�������·���±������ˮ������C����B�Ľṹ��ʽΪ![]() ��±��������ˮ�ⷴӦ������ΪNaOH��ˮ��Һ���ȣ�
��±��������ˮ�ⷴӦ������ΪNaOH��ˮ��Һ���ȣ�
(3) D�Ľṹ��ʽΪ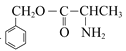 ��C��
��C��![]() �ڴ���������������D����Ϸ���ʽ��C�Ľṹ��ʽΪ
�ڴ���������������D����Ϸ���ʽ��C�Ľṹ��ʽΪ ����ѧ����ʽ��
����ѧ����ʽ�� +
+![]()
![]()
 +H2O��
+H2O��
(4)���ݷ�����D��E�ķ�Ӧ������ȡ����Ӧ��
(5)F�Ľṹ��ʽ��![]() ��
��
(6)��K�������ӳɷ�Ӧ���ڷ�����������ˮ�����X�ķ���ʽ���Լ�X������Ԫ����̼̼˫������ṹ��ʽ��![]() ��
��
�������ϳ�����������·���У���Yת��ΪG������F��Ӧ������ֱ����Y����ҪĿ���DZ���Y�е��Ȼ�����ϳ�·����C��D��ת��Ŀ����ͬ��

����Ŀ�����ΪԪ�����ڱ���һ���֣�����a��f�������ֶ���������Ԫ�ء����������գ�
a | b | c |
d | e | f |
��1������Ԫ���У�ԭ�Ӱ뾶������____(��Ԫ�ر��)��d��e��f����Ԫ�ص�ԭ�ӽṹ�ϵ���ͬ����___��
��2����a����̬�⻯���ˮ��Һ�ʼ��ԣ���a����̬�⻯��ĵ���ʽ��____������Ԫ���У�����������Ӧˮ�����������ǿ����____(��Ԫ�ط���)��
��3����fԪ�ص�ԭ��L���������M���������1������eԪ�صķǽ����Ա�fԪ�صķǽ�����_____(ѡ����ǿ������������)��
��4����bΪ�ǽ���Ԫ�أ��������ƶ���ȷ����____(ѡ����)��
��aһ���ǽ���Ԫ�� ��dһ���ǽ���Ԫ�� ��fһ���Ƿǽ���Ԫ��