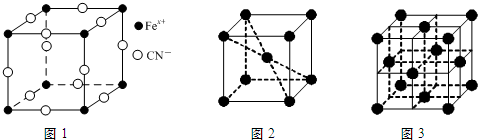
��Ŀ����
13����þ3%-5%��þ���Ͻ����ѳ�Ϊ�ִ����졢������������е�������ҵ����Ҫԭ���ϣ�����һ����֪����Ϊm1g��þ���Ͻ����ⶨ����þ��������������λͬѧ������������ֲ�ͬ��ʵ�鷽����ʵ�����1��þ���Ͻ�$\stackrel{������NaOH��Һ}{��}$��ַ�Ӧ��ⶨʣ���������m2g
ʵ�����2��þ���Ͻ�$\stackrel{��������}{��}$���ɵ������ڱ�״���µ����ΪVL��
��ش��������⣺
��1��ʵ�����1��
��þ������������$\frac{{m}_{2}}{m{\;}_{1}}$��100%��
�����ܽ⡢������ʹ�õIJ��������в�������©����
�۹��˺�ϴ�ӳ����IJ�����������������м�����ˮ���պý�û���������ã�ʹ����Ȼ���£��ظ�����2��3�Σ�
��������˵õ�����û��������ˮϴ�����κ�ɣ��ٲⶨʣ��������������þ��������������ƫ���ƫ����ƫС������Ӱ�족����
���� ��ʵ�����1������þ���Ͻ��е����ܽ���������������Һ�й���ϴ�ӵõ�����Ϊþ������þ�������ɼ����þ������������
�����������ܽ���˵�ʵ���������������Ҫ��������
�����ݹ�������ϴ�ӵĻ����������⣻
��������˵õ�����û��������ˮϴ�����κ��ɣ��ٲⶨʣ��������������溬�����ʻ�ʹ�ⶨ��������
��� �⣺����֪����Ϊm1g��þ���Ͻ����������������ڷ�Ӧ��õ���������Ϊm2g��Ϊþ��������������������=$\frac{{m}_{2}}{m{\;}_{1}}$��100%��
�ʴ�Ϊ��$\frac{{m}_{2}}{m{\;}_{1}}$��100%��ƫ��
�����������ܽ���˵�ʵ���������������Ҫ��������©�������������ձ���
�ʴ�Ϊ����������©����
�۹��˺�ϴ�ӳ����IJ�����������������м�����ˮ���պý�û���������ã�ʹ����Ȼ���£��ظ�����2��3�Σ�
�ʴ�Ϊ����������м�����ˮ���պý�û���������ã�ʹ����Ȼ���£��ظ�����2��3�Σ�
��������˵õ�����û��������ˮϴ�����κ��ɣ��ٲⶨʣ��������������溬�����ʻ�ʹ�ⶨ��������
�ʴ�Ϊ��ƫ��
���� ���⿼���˻�����ڳɷֵ�ʵ��ⶨ������ʵ����̷����жϣ�ʵ����Ʋ�֪�ͼ���Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

| A�� | ��������ƽ��ȡ2.50 g���������ȳ��ʧˮ������������0.90 g | |
| B�� | ����Ͳ�����ˮ���ռ��Ƶõ��������Ϊ50.28 mL | |
| C�� | ����ֻ250 mL������ƿ����0.1 mol•L-1500 mLNaOH��Һ | |
| D�� | ���³�ѹ�²��1 mol N2������Ϊ28 g |
| ʵ�� | ���ᣨ10%�����/mL | �¶�/�� | �������� |
| I | 2mL | 20 | / |
| II | 2mL | 20 | 10�α���MnSO4��Һ |
| III | 2mL | 30 | / |
| IV | 1mL | 20 | 1mL����ˮ |
��2������о������Ի�ѧ��Ӧ���ʵ�Ӱ�죬ʹ��ʵ���͢���I��IV��ʾ����ͬ��������о��¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬ʹ��ʵ���͢�
��3���Ա�ʵ��I��IV�������о�Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬ʵ��IV�м���1mL����ˮ��Ŀ����ȷ������ʵ����c��KMnO4����c��H2C2O4��Ũ�Ȳ������������䣮
| A�� | 2.0gH218O��D2O�Ļ����������������ΪNA | |
| B�� | 50g46%���Ҵ�ˮ��Һ�У���Hԭ������ĿΪ3NA | |
| C�� | 7.8gNa2O2����������ˮ�У���Ӧת�Ƶĵ�������ĿΪ0.2NA | |
| D�� | ���£�21.0g��ϩ�ͻ���ϩ�Ļ�������к��е�̼ԭ����Ϊ1.5NA |
| A�� | s��������Σ�p����������� | B�� | CuԪ����Ԫ�����ڱ���d�� | ||
| C�� | 12g������������0.8NA��Si-O�� | D�� | H2O��Oԭ�ӵ��ӻ���ʽ��sp3 |
| A�� | CH3��CH2��3CH3 | B�� | CH3CH2CH��CH3��CH3 | C�� | CH3CH2CH3 | D�� | CH3CH��CH3��CH3 |
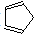 ���ϳɶ�ï���������ϩ��̼ԭ�ӵ��ӻ���ʽΪsp2��sp3��1mol�����ϩ�к��еĦҼ���ĿΪ11NA��
���ϳɶ�ï���������ϩ��̼ԭ�ӵ��ӻ���ʽΪsp2��sp3��1mol�����ϩ�к��еĦҼ���ĿΪ11NA��