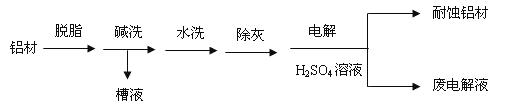
��Ŀ����
����Ŀ��A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��E�Ǿ��й�����ζ��Һ�塣A��B��C��D��E��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ���
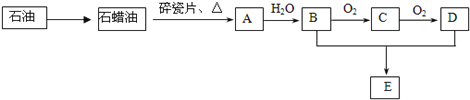
��ش��������⣺
��1����ҵ�ϣ���ʯ�ͻ��ʯ���͵ķ�����___________________��
��2����������ʯ���ͻ��A�Ĺ����е��м����֮һ������һ��ͬ���칹���к�������������CH3����������ͬ���칹��Ľṹ��ʽ�ǣ�___________________��D�����й����ŵ�������_______________��
��3��A��B��0.1 mol����ȫȼ������O2�������_______����״���£���
��4����ӦB��C�Ļ�ѧ����ʽΪ______________________��
��5����ӦB��D��E�Ļ�ѧ����ʽΪ______________________���÷�Ӧ�����ʱȽϻ�����ʵ����Ϊ����߸÷�Ӧ�����ʣ�ͨ����ȡ�Ĵ�ʩ��__________________��
���𰸡����� ![]() �Ȼ� 6.72L 2CH3CH2OH+O2
�Ȼ� 6.72L 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3COOH+CH3CH2OH
2CH3CHO+2H2O CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O ����Ũ���������������ȵ�
CH3COOCH2CH3+H2O ����Ũ���������������ȵ�
��������
A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��A����ϩ��E�Ǿ��й�����ζ��Һ�壬E��������������B���Ҵ���C����ȩ��D�����ᡣ�ݴ˽��
��1����ҵ�ϣ�ʯ��ͨ��������ʯ���͡�
��2������ķ���ʽ��C4H10,����һ��ͬ���칹���к�������������CH3����������ͬ���칹��Ľṹ��ʽ��![]() �� D��CH3COOH�������ŵ��������Ȼ���
�� D��CH3COOH�������ŵ��������Ȼ���
��3��A����ϩ��ȼ�յķ���ʽ��C2H4+3O2��2CO2+2H2O��B���Ҵ���ȼ�յķ���ʽ��C2H6O+3O2��2CO2+3H2O������0.1 mol��ϩ���Ҵ��Ļ������ȫȼ������0.3mol O2�������6.72L��
��4���Ҵ���ͭ�������������±���������Ϊ��ȩ����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��5���Ҵ������ᷢ��������Ӧ��������������ˮ����Ӧ�Ļ�ѧ����ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O���÷�Ӧ�����ʱȽϻ�����ʵ����Ϊ����߸÷�Ӧ�����ʣ�ͨ����ȡ�Ĵ�ʩ�У�����Ũ���������������ȵȡ�
CH3COOCH2CH3+H2O���÷�Ӧ�����ʱȽϻ�����ʵ����Ϊ����߸÷�Ӧ�����ʣ�ͨ����ȡ�Ĵ�ʩ�У�����Ũ���������������ȵȡ�

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�����Ŀ������п��ŨH2SO4����ڼ����·�Ӧ���ɻ�SO2��H2�Ļ�����壻п��ϡ���ᷴӦֻ��H2���ɡ���֪��Zn+2H2SO4(Ũ)![]() ZnSO4+2H2O+SO2�������м������о�С��ֱ�ʵ��̽����
ZnSO4+2H2O+SO2�������м������о�С��ֱ�ʵ��̽����
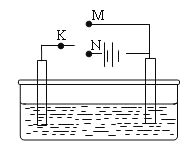
��1�����о�С�鰴��ͼʵ����֤п��Ũ���ᷴӦ��������SO2��H2��ȡ������Zn����b�У���a�м���100mL18.5mol��L��1��Ũ���ᣬ����һ��ʱ�䷴Ӧ��Zn��ȫ�ܽ⡣

����д�������ƣ�a___________��b___________��
���о�С����Ϊ�����ܲ��������������ǣ�_____________________��
��װ��D�м�����Լ���__________��
��U��G������Ϊ__________��
����ͬѧ��ΪA��B��Ӧ����ͼ�еļ�װ�ã���װ�õ�����Ϊ__________��
��֤����Ӧ����SO2��H2��ʵ��������______________________________��
��2�����о�С��Ϊ��̽��п��ϡ���ᷴӦ�����е����ʼ������ı仯����������ʵ�飬����Ӱ�췴Ӧ���ʵ����ء�

ʵ��ʱ���ӶϿ�K��ʼ��ÿ���1���ӣ�����Ͽ���պ�K������������ÿ1�����ڴ�a��������ˮ�������õ���ˮ�������±���ʾ��
1����ˮ�������Ͽ�K�� | 34 | 59 | 86 | 117 | �� | 102 |
1����ˮ�������պ�K�� | 58 | 81 | 112 | 139 | �� | 78 |
������Ӧ�����е�ˮ��������ش�
�� ��ˮ����58��34��81��59��˵���ڷ�Ӧ���ڣ��պ�Kʱ�ȶϿ�Kʱ�ķ�Ӧ���ʿ죬��Ҫԭ����________��
�� ��ˮ����102��78��˵���ڷ�Ӧ���ڣ��Ͽ�Kʱ�ķ�Ӧ���ʿ��ڱպ�Kʱ�ķ�Ӧ���ʣ���Ҫԭ����______��