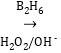��Ŀ����
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵����ȷ����
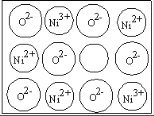
A.0.5 mol�ۻ�( As4S4���ṹΪ![]() )�к���NA��S-S��
)���NA��S-S��
B.��1mol /L��NH4NO3��Һ�μӰ�ˮʹ��Һ�����ԣ���1L����Һ��NH4+����ĿΪNA
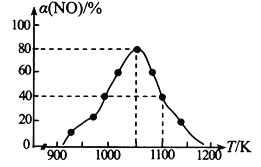
C.��״���£�33.6 L�������к��з�ԭ�ӵ���ĿΪ1.5NA
D.�����£���1 mol CH4��1 mol Cl2��Ϲ��գ�ʹ���ַ�Ӧ����������ķ�����ΪNA
���𰸡�B
��������
A. As4S4�У�AsΪ��VA��Ԫ�أ�SΪ��VIA��Ԫ�أ�As����������S�����������ʰ���ΪS�������ۻ��в�����S-S����A����
B. ��1mol /L��NH4NO3��Һ�μӰ�ˮ���ɵ���غ��֪![]() ����Ϊ��Һ�����ԣ���
����Ϊ��Һ�����ԣ���![]() ������
������![]() ����1L����Һ��NH4+����Ŀ���������ͬΪNA��B��ȷ��
����1L����Һ��NH4+����Ŀ���������ͬΪNA��B��ȷ��
C. ��״���£�������Ϊ����̬����������㣬C����
D. �ڹ��������£���1molCH4��1molCl2��ϳ�ַ�Ӧ�������������㣬�ɷ����ಽȡ����Ӧ����������CH3Cl��CH2Cl2��CHCl3��CCl4��HCl�ȣ�����ȷ�����ɵ������������D����
�ʴ�ѡB��

��ϰ��ϵ�д�
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
�����Ŀ