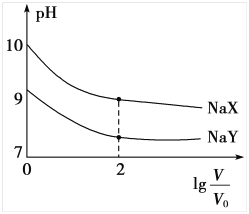
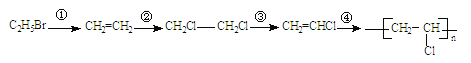ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΗΜ¬μΥαΘ®Ζ¥ ΫΕΓœ©ΕΰΥαΘ©”κFe2+–Έ≥…ΒΡ≈δΚœΈοΓΣΓΣΗΜ¬μΥαΧζ”÷≥ΤΓΑΗΜ―ΣΧζΓ±Θ§Ω…”Ο”Ύ÷ΈΝΤ»±Χζ–‘ΤΕ―ΣΓΘ“‘œ¬ «Κœ≥…ΗΜ¬μΥαΧζΒΡ“Μ÷÷ΙΛ“’¬ΖœΏΘΚ
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©AΒΡΜ·―ßΟϊ≥ΤΈΣ____________Θ§ΔΌΒΡΖ¥”Πάύ–ΆΈΣ____________Θ§ΔΎΒΡΖ¥”Πάύ–ΆΈΣ____________ΓΘ
Θ®2Θ©ΗΜ¬μΥαΒΡΫαΙΙΦρ ΫΈΣ______________ΓΘ
Θ®3Θ©Φλ―ιΗΜ―ΣΧζ÷– «ΖώΚ§”–Fe3+ΒΡ Β―ι≤ΌΉς≤Ϋ÷η «________________________________________ΓΘ
Θ®4Θ©–¥≥ωœ¬Ν–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
ΔΌ_____________________________________________________________ΓΘ
Δή______________________________________________________________ΓΘ
Δό_______________________________________________________________ΓΘ
Θ®5Θ©ΗΜ¬μΥαΈΣΕΰ‘Στ»ΥαΘ§1molΗΜ¬μΥα”κΉψΝΩ±ΞΚΆNaHCO3»ή“ΚΖ¥”ΠΩ…Ζ≈≥ω____ L CO2Θ®±ξΩωΘ©ΘΜΗΜ¬μΥαΒΡΆ§Ζ÷“λΙΙΧε÷–Θ§Ά§ΈΣΕΰ‘Στ»ΥαΒΡΜΙ”–____________________________________Θ®–¥≥ωΫαΙΙΦρ ΫΘ©ΓΘ
ΓΨ¥πΑΗΓΩΜΖΦΚΆι »Γ¥ζΖ¥”Π œϊ»ΞΖ¥”Π ![]() »Γ…ΌΝΩΗΜ―ΣΧζΘ§Φ”»κœΓΝρΥα»ήΫβΘ§‘ΌΒΈΦ”KSCN»ή“ΚΘ§»τ»ή“Κœ‘―ΣΚλ…ΪΘ§‘ρ≤ζΤΖ÷–Κ§”–Fe3+ΘΜΖ¥÷°Θ§‘ρΈό
»Γ…ΌΝΩΗΜ―ΣΧζΘ§Φ”»κœΓΝρΥα»ήΫβΘ§‘ΌΒΈΦ”KSCN»ή“ΚΘ§»τ»ή“Κœ‘―ΣΚλ…ΪΘ§‘ρ≤ζΤΖ÷–Κ§”–Fe3+ΘΜΖ¥÷°Θ§‘ρΈό 

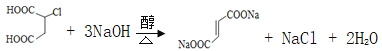 44.8
44.8 ![]()
ΓΨΫβΈωΓΩ
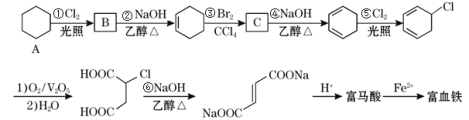
AΈΣΜΖΦΚΆιΘ§ΚΆ¬»Τχ‘ΎΙβ’’ΒΡΧθΦΰœ¬ΖΔ…ζ»Γ¥ζΖ¥”ΠΘ§…ζ≥…¬»¥ζΧΰ![]() ΘΜ
ΘΜ![]() ‘ΎNaOHΒΡ¥Φ»ή“ΚΦ”»»ΧθΦΰœ¬ΖΔ…ζœϊ»ΞΖ¥”ΠΘ§“ΐ»κΧΦΧΦΥΪΦϋΘ§…ζ≥…ΜΖΦΚœ©ΘΜΜΖΦΚœ©Φ”≥…Θ§…ζ≥…1Θ§2-ΕΰδεΜΖΦΚΆιΘ§‘Όœϊ»Ξ…ζ≥…
‘ΎNaOHΒΡ¥Φ»ή“ΚΦ”»»ΧθΦΰœ¬ΖΔ…ζœϊ»ΞΖ¥”ΠΘ§“ΐ»κΧΦΧΦΥΪΦϋΘ§…ζ≥…ΜΖΦΚœ©ΘΜΜΖΦΚœ©Φ”≥…Θ§…ζ≥…1Θ§2-ΕΰδεΜΖΦΚΆιΘ§‘Όœϊ»Ξ…ζ≥…![]() Θ§
Θ§![]() ”ꬻΤχΖΔ…ζ»Γ¥ζΖ¥”Π…ζ≥…
”ꬻΤχΖΔ…ζ»Γ¥ζΖ¥”Π…ζ≥…![]() Θ§
Θ§![]() ΖΔ…ζ―θΜ·Ζ¥”Π…ζ≥…
ΖΔ…ζ―θΜ·Ζ¥”Π…ζ≥… Θ§‘ΌΖΔ…ζœϊ»ΞΖ¥”ΠΓΔ÷–ΚΆΖ¥”ΠΒΟΒΫ
Θ§‘ΌΖΔ…ζœϊ»ΞΖ¥”ΠΓΔ÷–ΚΆΖ¥”ΠΒΟΒΫ Θ§Ϋχ––ΥαΜ·ΒΟΒΫΗΜ¬μΥαΈΣΘΚ
Θ§Ϋχ––ΥαΜ·ΒΟΒΫΗΜ¬μΥαΈΣΘΚ![]() ΓΘ
ΓΘ
Θ®1Θ©AΒΡΜ·―ßΟϊ≥ΤΈΣΜΖΦΚΆιΘ§ΔΌΒΡΖ¥”Πάύ–ΆΈΣ»Γ¥ζΖ¥”ΠΘΜΔΎΒΡΖ¥”Πάύ–ΆΈΣœϊ»ΞΖ¥”ΠΘ§
Ι ¥πΑΗΈΣΘΚΜΖΦΚΆιΘΜ»Γ¥ζΖ¥”ΠΘΜœϊ»ΞΖ¥”ΠΘΜ
Θ®2Θ©”……œ ωΖ÷ΈωΩ…÷ΣΘ§ΗΜ¬μΥαΒΡΫαΙΙΦρ ΫΈΣΘΚ![]() Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
Θ®3Θ©Φλ―ιΗΜ―ΣΧζ÷– «ΖώΚ§”–Fe3+Θ§”ωΒΫKSCN‘ρœ‘―ΣΚλ…ΪΘ§»Γ…ΌΝΩΗΜ―ΣΧζΘ§Φ”»κœΓΝρΥα»ήΫβΘ§‘ΌΒΈΦ”KSCN»ή“ΚΘ§»τ»ή“Κœ‘―ΣΚλ…ΪΘ§‘ρ≤ζΤΖ÷–Κ§”–Fe3+ΘΜΖ¥÷°Θ§‘ρΈόΘΜ
Θ®4Θ©ΗυΨίΖ÷ΈωΩ…÷ΣΘ§ΔΌ ΘΜ
ΘΜ
Δή ΘΜ
ΘΜ
Δό
Θ®5Θ©ΗΜ¬μΥαΈΣΕΰ‘Στ»ΥαΘ§1molΗΜ¬μΥα”κΉψΝΩ±ΞΚΆNaHCO3»ή“ΚΖ¥”ΠΩ…Ζ≈≥ω2mol CO2Θ§±ξΩωœ¬…ζ≥…Εΰ―θΜ·ΧΦΒΡΧεΜΐΈΣ2molΓΝ22.4L/mol=44.8LΘ§ΗΜ¬μΥαΒΡΆ§Ζ÷“λΙΙΧε÷–Θ§Ά§ΈΣΕΰ‘Στ»ΥαΒΡΜΙ”–![]() Θ§Ι ¥πΑΗΈΣΘΚ44.8ΘΜ
Θ§Ι ¥πΑΗΈΣΘΚ44.8ΘΜ![]() ΘΜ
ΘΜ

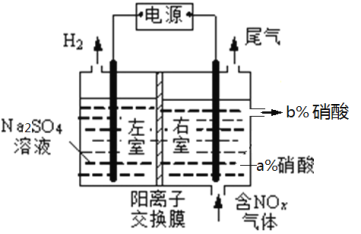
ΓΨΧβΡΩΓΩ”ΈάκΧ§ΒΣ≥ΤΈΣΕη–‘ΒΣΘ§”ΈάκΧ§ΒΣΉΣΜ·ΈΣΜ·ΚœΧ§ΒΣ≥Τ÷°ΈΣΒΣΒΡΜνΜ·Θ§‘ΎΒΣΒΡ―≠ΜΖœΒΆ≥÷–Θ§ΒΣΒΡΙΐΝΩΓΑΜνΜ·Γ±Θ§‘ρΜνΜ·ΒΣΩΣ Φœρ¥σΤχΚΆΥ°ΧεΙΐΝΩ«®“ΤΘ§ΒΣΒΡ―≠ΜΖΤΫΚβ±Μ¥ρΤΤΘ§ΒΦ÷¬»Ϊ«ρΜΖΨ≥Έ ΧβΓΘ
ΔώΘ° ΒΣΒΡΜνΜ·
ΙΛ“ΒΚœ≥…Α± «ΒΣΒΡΜνΜ·÷Ί“ΣΆΨΨΕ÷°“ΜΘ§‘Ύ“ΜΕ®ΧθΦΰœ¬Θ§ΫΪN2 ΚΆ H2 Ά®»κΒΫΧεΜΐΈΣ0.5LΒΡΚψ»ί»ίΤς÷–Θ§Ζ¥”ΠΙΐ≥Χ÷–ΗςΈο÷ ΒΡΈο÷ ΒΡΝΩ±δΜ·»γΆΦΥυ ΨΘΚ

Θ®1Θ©10minΡΎ”ΟNH3±μ ΨΗΟΖ¥”ΠΒΡΤΫΨυΥΌ¬ Θ§vΘ®NH3Θ©=____________ΓΘ
Θ®2Θ©‘ΎΒΎ10minΚΆΒΎ25minΗΡ±δΒΡΧθΦΰΩ…ΡήΖ÷±π «_________ΓΔ________Θ®ΧνΉ÷ΡΗΘ©ΓΘ
A.Φ”ΝΥ¥ΏΜ·ΦΝ B. …ΐΗΏΈ¬Ε» C. ‘ωΦ”NH3ΒΡΈο÷ ΒΡΝΩ
D.―ΙΥθΧεΜΐ E.Ζ÷άκ≥ωΑ±Τχ
Θ®3Θ©œ¬Ν–ΥΒΖ®ΡήΥΒΟςΗΟΩ…ΡφΖ¥”Π¥οΒΫΤΫΚβΉ¥Χ§ΒΡ «__________Θ®ΧνΉ÷ΡΗΘ©ΓΘ
A. »ίΤς÷–ΤχΧεΟήΕ»≤Μ±δ B. »ίΤς÷–―Ι«Ω≤Μ±δ
C. 3v(H2)’ΐ=2v(NH3)Ρφ D. N2ΓΔH2ΓΔNH3Ζ÷Ή” ΐ÷°±»ΈΣ1ΓΟ3ΓΟ2
ΔρΘ°¥ΏΜ·ΉΣΜ·ΈΣΕη–‘ΒΣ “―÷ΣΘΚSO2ΓΔCOΓΔNH3Β»ΕΦΩ…“‘¥ΏΜ·ΜΙ‘≠ΒΣ―θΜ·Έο…ζ≥…Εη–‘ΒΣΓΘ
Θ®4Θ©‘Ύ250CΓΔ101KPa ±Θ§N2(g)+3H2(g) ![]() 2NH3(g) ΓςH1= -92.4kJ/mol.
2NH3(g) ΓςH1= -92.4kJ/mol.
2H2(g)+O2(g)=2H2O(l) ΓςH2= -571.6 kJ/mol
N2(g)+O2(g)=2NO(g) ΓςH3= +180kJ/mol
‘ρNO”κNH3Ζ¥”Π…ζ≥…Εη–‘ΒΣΒΡ»»Μ·―ßΖΫ≥Χ Ϋ______________________________ΓΘ
Θ®5Θ©‘Ύ”–―θΧθΦΰœ¬Θ§–¬–Ά¥ΏΜ·ΦΝMΡή¥ΏΜ·CO”κNOxΖ¥”Π…ζ≥…N2ΓΘœ÷œρΡ≥Οή±’»ίΤς÷–≥δ»κΒ»Έο÷ ΒΡΝΩ≈®Ε»ΒΡNO2ΚΆCOΤχΧεΘ§Έ§≥÷ΚψΈ¬Κψ»ίΘ§‘Ύ¥ΏΜ·ΦΝΉς”Οœ¬ΖΔ…ζΖ¥”ΠΘΚ
4CO(g)+2NO2(g)![]() N2(g)+4CO2(g) ΓςHΘΦ0Θ§œύΙΊ ΐΨί»γœ¬ΘΚ
N2(g)+4CO2(g) ΓςHΘΦ0Θ§œύΙΊ ΐΨί»γœ¬ΘΚ
0min | 5min | 10min | 15min | 20min | |
c(NO2) /molΓΛL-1 | 2.0 | 1.7 | 1.56 | 1.5 | 1.5 |
c(N2) /molΓΛL-1 | 0 | 0.15 | 0.22 | 0.25 | 0.25 |
ΔΌΦΤΥψ¥ΥΈ¬Ε»œ¬ΒΡΜ·―ßΤΫΚβ≥Θ ΐK=______Θ§
ΔΎ Β―ι “ΡΘΡβΒγΫβΖ®Έϋ ’NOxΉΑ÷Ο»γΆΦΘ§Θ®ΆΦ÷–ΒγΦΪΨυΈΣ ·ΡΪΒγΦΪΘ©ΓΘ»τ”ΟNO2ΤχΧεΫχ––ΡΘΡβΒγΫβΖ®Έϋ ’ Β―ιΘ®a<bΘ©Θ§ΒγΫβ ±NO2ΖΔ…ζΖ¥”ΠΒΡΒγΦΪΖ¥”Π ΫΘΚ_________________________________ΓΘ